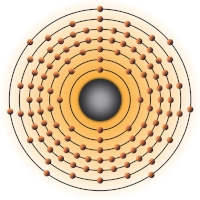
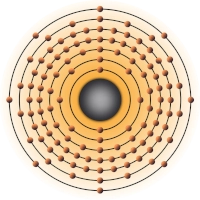
- 2
- 2
- 8
- 2
- 8
- 8
- 2
- 8
- 18
- 8
- 2
- 8
- 18
- 18
- 8
- 2
- 8
- 18
- 18
- 9
- 2
- 2
- 8
- 18
- 19
- 9
- 2
- 2
- 8
- 18
- 21
- 8
- 2
- 2
- 8
- 18
- 22
- 8
- 2
- 2
- 8
- 18
- 23
- 8
- 2
- 2
- 8
- 18
- 24
- 8
- 2
- 2
- 8
- 18
- 25
- 8
- 2
- 2
- 8
- 18
- 25
- 9
- 2
- 2
- 8
- 18
- 27
- 8
- 2
- 2
- 8
- 18
- 28
- 8
- 2
- 2
- 8
- 18
- 29
- 8
- 2
- 2
- 8
- 18
- 30
- 8
- 2
- 2
- 8
- 18
- 31
- 8
- 2
- 2
- 8
- 18
- 32
- 8
- 2
- 2
- 8
- 18
- 32
- 9
- 2
- 2
- 8
- 18
- 32
- 18
- 8
- 2
- 8
- 18
- 32
- 18
- 9
- 2
- 2
- 8
- 18
- 32
- 18
- 10
- 2
- 2
- 8
- 18
- 32
- 20
- 9
- 2
- 2
- 8
- 18
- 32
- 21
- 9
- 2
- 2
- 8
- 18
- 32
- 22
- 9
- 2
- 2
- 8
- 18
- 32
- 24
- 8
- 2
- 2
- 8
- 18
- 32
- 25
- 8
- 2
- 2
- 8
- 18
- 32
- 25
- 9
- 2
- 2
- 8
- 18
- 32
- 27
- 8
- 2
- 2
- 8
- 18
- 32
- 28
- 8
- 2
- 2
- 8
- 18
- 32
- 29
- 8
- 2
- 2
- 8
- 18
- 32
- 30
- 8
- 2
- 2
- 8
- 18
- 32
- 31
- 8
- 2
- 2
- 8
- 18
- 32
- 32
- 8
- 2
- 2
- 8
- 18
- 32
- 32
- 8
- 3
- 2
- 8
- 18
- 32
- 32
- 15
- 2
- 2
- 8
- 18
- 32
- 32
- 17
- 1
- 2
- 8
- 18
- 32
- 32
- 17
- 2
- 2
- 8
- 18
- 32
- 32
- 18
- 2
- 2
- 8
- 18
- 32
- 32
- 18
- 3
- 2
- 8
- 18
- 32
- 32
- 18
- 4
- 2
- 8
- 18
- 32
- 32
- 18
- 5
- 2
- 8
- 18
- 32
- 32
- 18
- 6
- 2
- 8
- 18
- 32
- 32
- 18
- 7
- 2
- 8
- 18
- 32
- 32
- 18
- 8