Valency of first 30 elements of periodic table is mentioned in the table below.
| Atomic Number | Valency of Elements | Valency |
| 1 | Valency of Hydrogen (H) | 1 |
| 2 | Valency of Helium (He) | 0 |
| 3 | Valency of Lithium (Li) | 1 |
| 4 | Valency of Beryllium (Be) | 2 |
| 5 | Valency of Boron (B) | 3 |
| 6 | Valency of Carbon (C) | 4 |
| 7 | Valency of Nitrogen (N) | 3 |
| 8 | Valency of Oxygen (O) | 2 |
| 9 | Valency of Fluorine (F) | 1 |
| 10 | Valency of Neon (Ne) | 0 |
| 11 | Valency of Sodium (Na) | 1 |
| 12 | Valency of Magnesium (Mg) | 2 |
| 13 | Valency of Aluminum (Al) | 3 |
| 14 | Valency of Silicon (Si) | 4 |
| 15 | Valency of Phosphorus (P) | 3 |
| 16 | Valency of Sulfur (S) | 2 |
| 17 | Valency of Chlorine (Cl) | 1 |
| 18 | Valency of Argon (Ar) | 0 |
| 19 | Valency of Potassium (K) | 1 |
| 20 | Valency of Calcium (Ca) | 2 |
| 21 | Valency of Scandium (Sc) | 3 |
| 22 | Valency of Titanium (Ti) | 4 |
| 23 | Valency of Vanadium (V) | 5, 4 |
| 24 | Valency of Chromium (Cr) | 2 |
| 25 | Valency of Manganese (Mn) | 7, 4, 2 |
| 26 | Valency of Iron (Fe) | 2, 3 |
| 27 | Valency of Cobalt (Co) | 3, 2 |
| 28 | Valency of Nickel (Ni) | 2 |
| 29 | Valency of Copper (Cu) | 2, 1 |
| 30 | Valency of Zinc (Zn) | 2 |
Well, these are the valencies of first 30 elements of Periodic table.
But do you know;
- What exactly is the valency?
- Why do some elements have more than one valency?
Don’t worry, I’ll explain this in just a few seconds.
Quick look at: What is valency?
Valency is the maximum number of electrons that an atom loses, gains or shares to become stabilized during a chemical reaction.
For example;
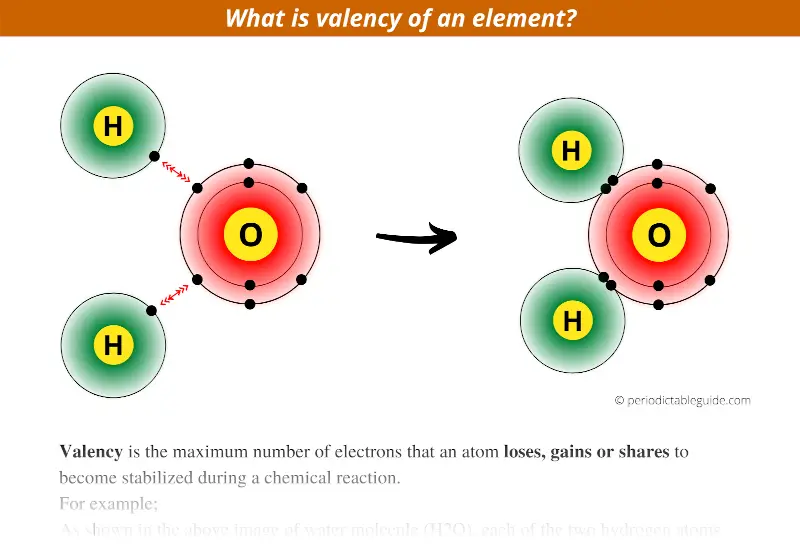
As shown in the above image of water molecule (H2O), each of the two hydrogen atoms (H) shares one electron with the oxygen atom.
In other words, the hydrogen atom loses one electron to become stable.
So the number one indicates the valency or the combining capacity of the Hydrogen atom in H2O molecule.
Similarly,
Let’s understand the same thing from oxygen’s side.
See in above image only, you can see that the oxygen atom gains two electrons to form a stable atom. (Oxygen gains one electron from 1st hydrogen and other electron from 2nd hydrogen)
Thus for oxygen, the valency is two or we can say that combining capacity of oxygen is two.
Why do some elements have more than one valency?
The short answer: The elements have more than one valency because they have more than one combining capacity.
For example,
Example #1
In CuO (cupric oxide), one copper atom is bounded with one oxygen atom.
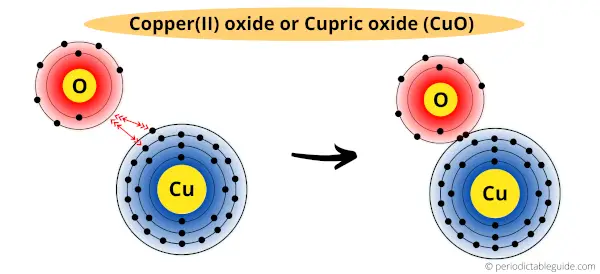
Copper has two electrons in its outermost orbit, and oxygen has 6 electrons in its outermost orbit.
Copper loses its two electrons to the oxygen atom and becomes stabilized.
Thus in CuO, copper has a valency of two.
Example #2
In Cu2O (cuprous oxide), two copper atoms are bounded with one oxygen atom.
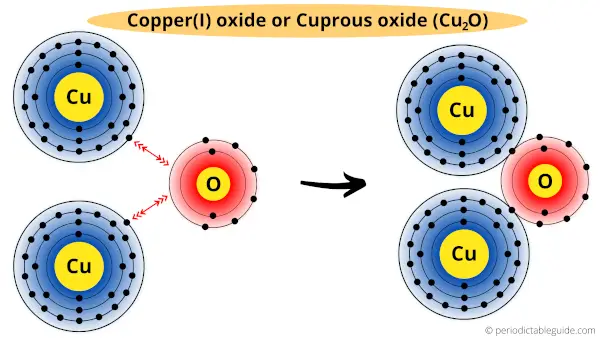
Here in Cu2O, each of the copper atoms loses their one electron to the oxygen atom.
In other words, an oxygen atom receives a one-one electron from both the copper atoms.
(Or we can also say that, copper has a combining capacity of one in Cu2O)
Hence, the valency of copper is one in Cu2O.
Outcome from Example #1 and #2
So we have seen from the above example #1 that copper loses two electrons (i.e copper has a valency of two)
While in the second example, we saw that copper loses only one electron (i.e copper has a valency of one)
From the above two examples, we saw that the valency of copper is one as well as two.
Thus, being the same element it could have different valency.
This is known as variable valency.
Why variable valency occurs?
Variable valency occurs due to the different possibility of sharing or losing or gaining electrons based on the combination of different different elements.
In other words, variable valency occurs because one element combines with another in one or the other way.
For example,
We have seen that in CuO, one copper atom combines with the oxygen atom by sharing two electrons.
While in Cu2O, two copper atoms combine with oxygen by sharing one electron.
This results in variable valency of the same element.
Also see:
1). Valence electrons of first 30 elements
2). Electronic configuration of first 30 elements
3). 118 elements and their symbols and atomic number
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Summary
So in the very beginning, you have seen the first 30 elements of the Periodic table with valency.
Then we discussed the brief concept of valency and the reason why some elements have variable valency.
I showed you two examples, one of CuO and other of Cu2O and explained the concept of variable valency.
I hope you have found this article helpful.
If you have any doubts, feel free to ask me in the comments below.
Also let me know, has this article helped you or not?
Suggested Important topics for you:
- Periodic table of elements (Detailed guide + HD image)
- Periodic table with atomic radius values
- Periodic table of elements with names and symbols
- Periodic table with electron shells
- Metals on the periodic table
- Nonmetals on the periodic table
- Metalloids on periodic table
- Halogens on periodic table
- All Periodic trends in periodic table
