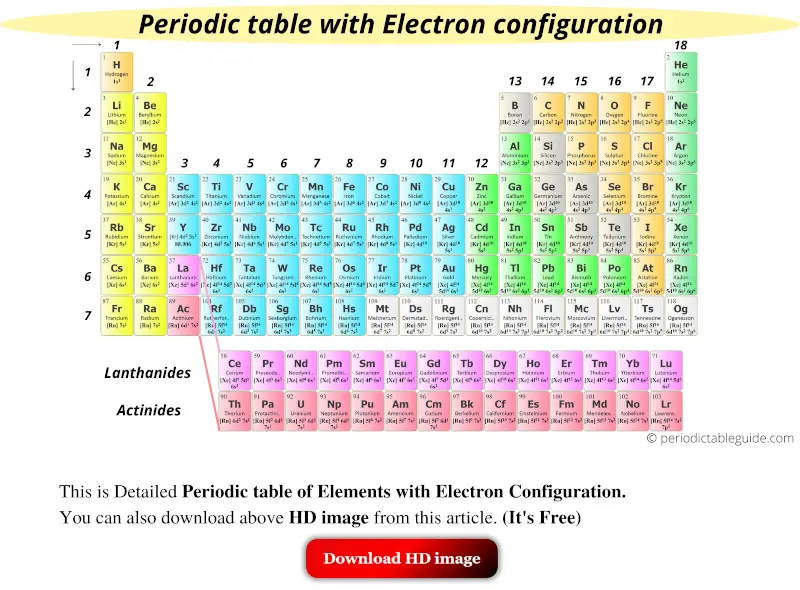
This is the detailed Periodic table with electron configuration where you can find the electron configuration of all the elements written in the table itself.
But,
A few questions for you…
- Do you know what exactly is the electron configuration?
- Why is electron configuration important?
- What does 1s2 2s2 2p6 mean?
Don’t worry, all these things will be cleared in this article.
+
Bonus: You will also get the HD image of the above Periodic table.
Let’s dive straight into it.
What exactly is the Electron Configuration?
Electron configuration is the structure which describes the arrangement of electrons around the nucleus of an atom.
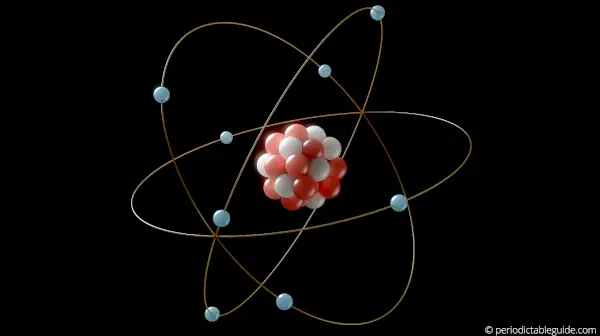
This is how the electrons are arranged around the nucleus of an atom. In simple words, if we know the electron configuration of an element, then we can easily get the idea of how the electrons are arranged around the atom.
What are orbitals?
There are few regions around the nucleus where the probability of finding electrons is maximum. Such regions are known as orbitals.

For example, here you can see that the blue and yellow color regions around the nucleus show a maximum probability of finding the electrons.
These regions around the nucleus are known as orbitals or subshells.
There are total 4 orbitals.
- s-orbitals
- p-orbitals
- d-orbitals
- f-orbitals
Why is Electron configuration important?
Electronic configuration is important in the following ways.
- Electron configuration is very important to know where the electrons are located around the atoms.
- Number of electrons present in s, p, d and f orbitals can be found using electron configuration.
- Through electronic configuration, the chemical properties of elements can be predicted by knowing their valence electrons.
What does 1s2 2s2 2p6 mean?
1s2 2s2 2p6 simply indicates the following things.
1s2 2s2 2p6
- The number 1, 2 and 2 (in red color) indicates the Principal quantum number.
1 represents the 1st shell, and
2 represents the 2nd shell.
1s2 2s2 2p6
- The s and p indicates that the electrons are lying in that orbitals.
1s2 2s2 2p6
- The 2, 2 and 6 (in red color) are the number of electrons present in that particular orbitals
- Here, 2 electrons are in s-orbitals of 1st energy shell.
- Similarly, other 2 electrons are in s-orbitals of the 2nd energy shell, and
- 6 electrons are in p-orbitals.
List of elements with electron configuration
| Element | Electron configuration |
| 1 | Hydrogen (H) | 1s1 |
| 2 | Helium (He) | 1s2 |
| 3 | Lithium (Li) | [He] 2s1 |
| 4 | Beryllium (Be) | [He] 2s2 |
| 5 | Boron (B) | [He] 2s2 2p1 |
| 6 | Carbon (C) | [He] 2s2 2p2 |
| 7 | Nitrogen (N) | [He] 2s2 2p3 |
| 8 | Oxygen (O) | [He] 2s2 2p4 |
| 9 | Fluorine (F) | [He] 2s2 2p5 |
| 10 | Neon (Ne) | [He] 2s2 2p6 |
| 11 | Sodium (Na) | [Ne] 3s1 |
| 12 | Magnesium (Mg) | [Ne] 3s2 |
| 13 | Aluminum (Al) | [Ne] 3s2 3p1 |
| 14 | Silicon (Si) | [Ne] 3s2 3p2 |
| 15 | Phosphorus (P) | [Ne] 3s2 3p3 |
| 16 | Sulfur (S) | [Ne] 3s2 3p4 |
| 17 | Chlorine (Cl) | [Ne] 3s2 3p5 |
| 18 | Argon (Ar) | [Ne] 3s2 3p6 |
| 19 | Potassium (K) | [Ar] 4s1 |
| 20 | Calcium (Ca) | [Ar] 4s2 |
| 21 | Scandium (Sc) | [Ar] 3d1 4s2 |
| 22 | Titanium (Ti) | [Ar] 3d2 4s2 |
| 23 | Vanadium (V) | [Ar] 3d3 4s2 |
| 24 | Chromium (Cr) | [Ar] 3d5 4s1 |
| 25 | Manganese (Mn) | [Ar] 3d5 4s2 |
| 26 | Iron (Fe) | [Ar] 3d6 4s2 |
| 27 | Cobalt (Co) | [Ar] 3d7 4s2 |
| 28 | Nickel (Ni) | [Ar] 3d8 4s2 |
| 29 | Copper (Cu) | [Ar] 3d10 4s1 |
| 30 | Zinc (Zn) | [Ar] 3d10 4s2 |
I hope you have clearly understood the detailed Periodic table with electron configuration.
If you have any queries, feel free to ask me in the comments below.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Suggested Important articles for you:
- Periodic table of elements (Detailed guide + HD image)
- Periodic table with metals
- Periodic table with nonmetals
- Periodic table with metalloids
- Periodic table with halogens
- Periodic table with noble gases
- Periodic table showing alkali metals
- Periodic table showing alkaline earth metals
- Periodic table with transition metals
- Periodic table with inner transition metals
- Periodic trends (Trends in periodic table)
