The periods on the Periodic table represents the number of shells (or orbits) of an atom.
For example,
Period 1 represents that the elements lying in that period have only 1 orbit.
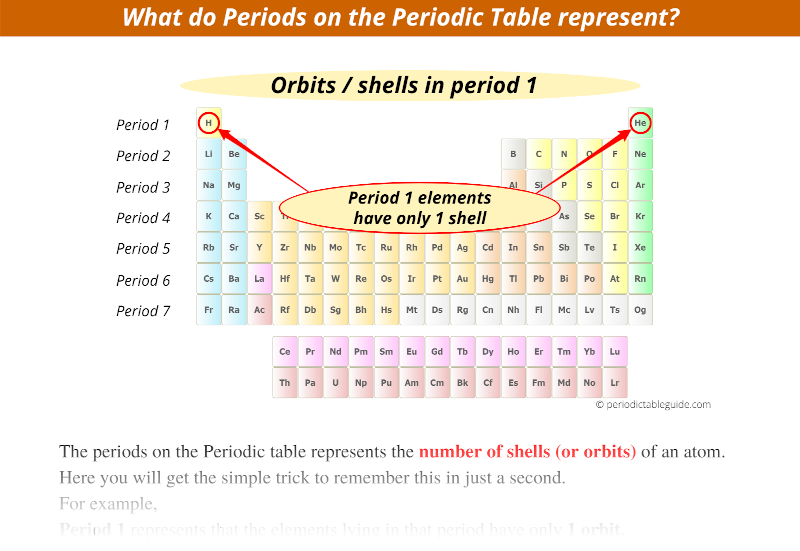
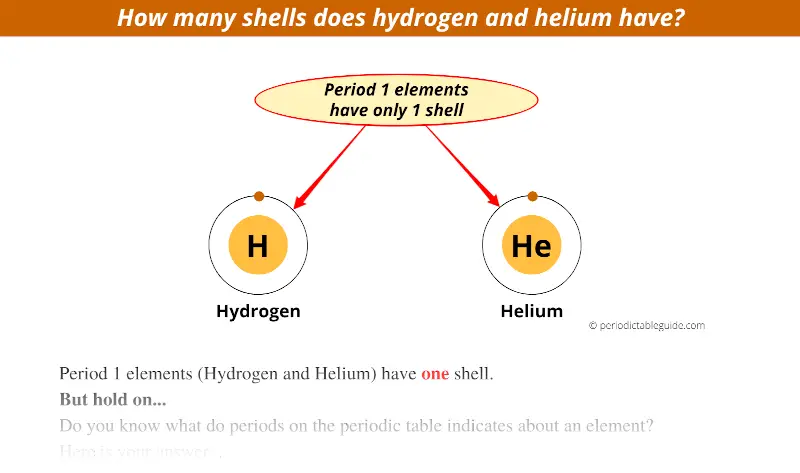
There are 2 elements in the first period i.e hydrogen (H) and helium (He).
These elements have only 1 electron orbit.
Similarly, period 2 represents that the elements lying in that period have 2 orbits.
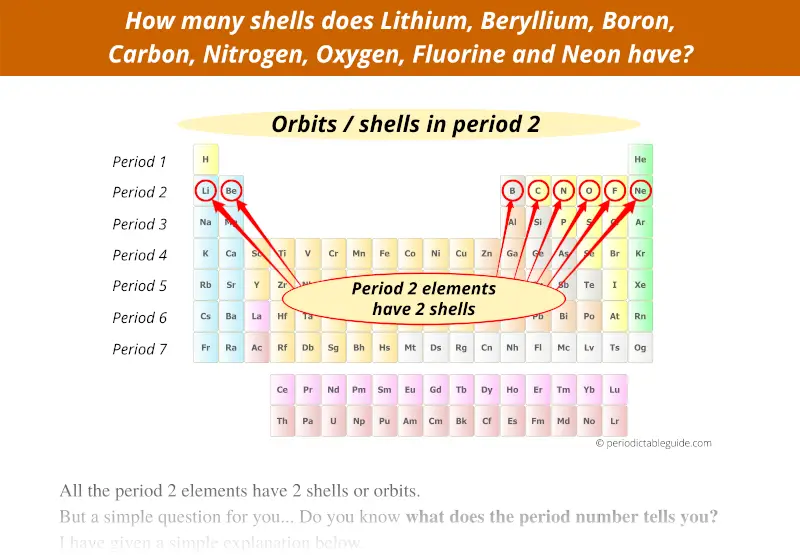
Here you can see that all the 8 elements of the second period have 2 orbits.
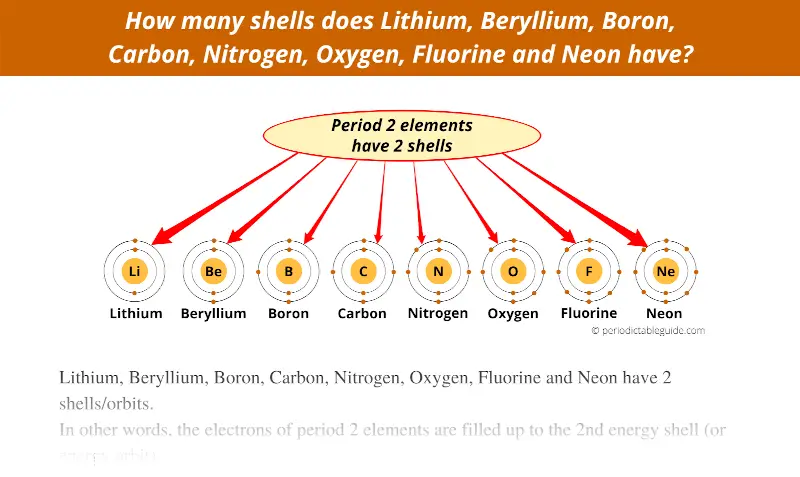
In other words, the electrons of period 2 elements are filled up to the 2nd energy shell (or energy orbit).
Similarly,
Period 3 elements have 3 orbits.
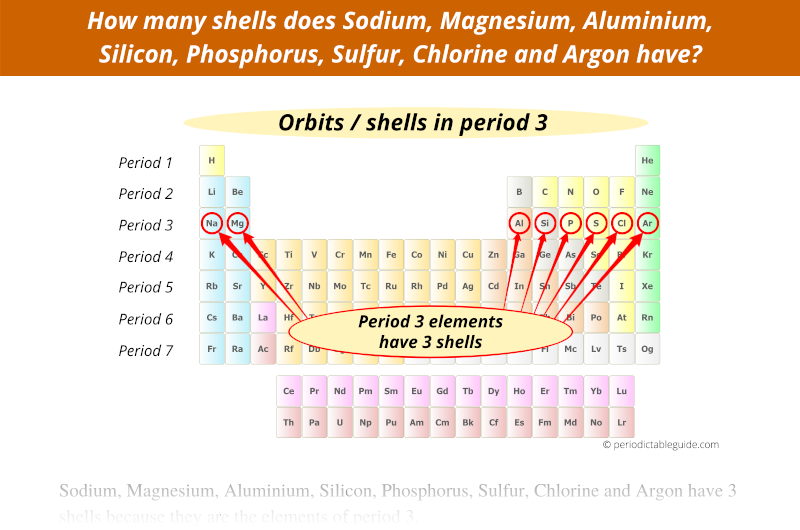
Period 4 elements have 4 orbits.
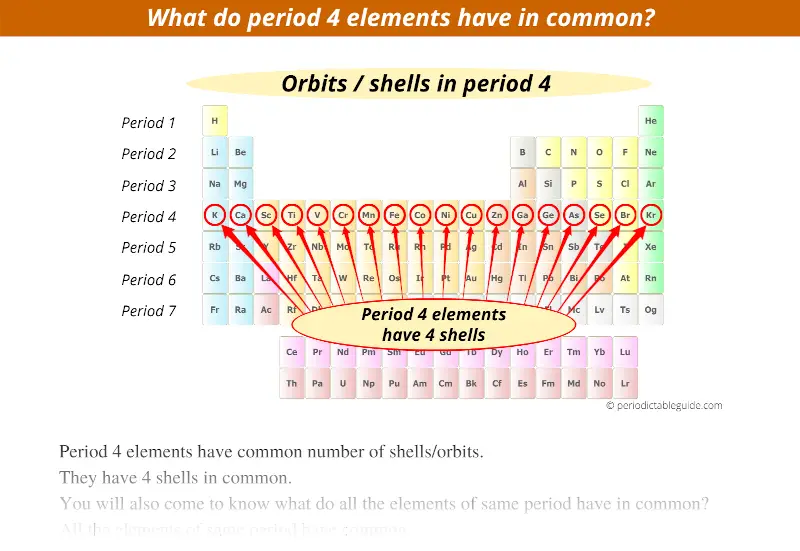
Period 5 elements have 5 orbits.
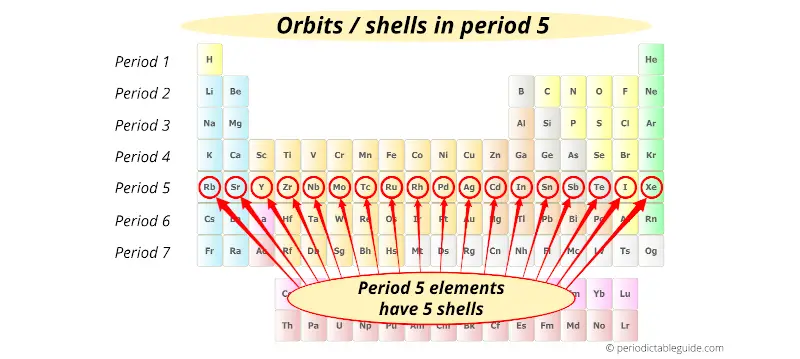
Period 6 elements have 6 orbits.
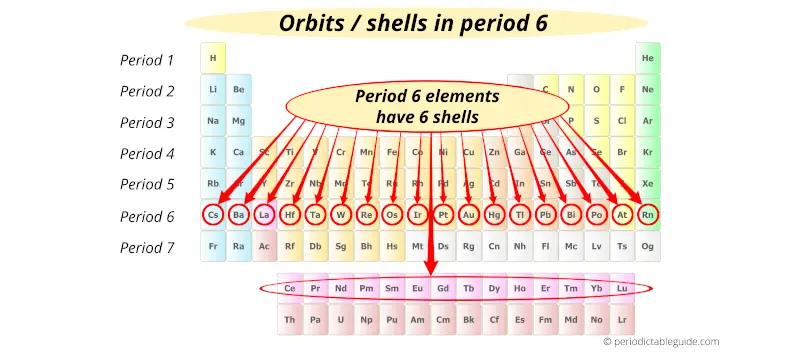
Period 7 elements have 7 orbits.
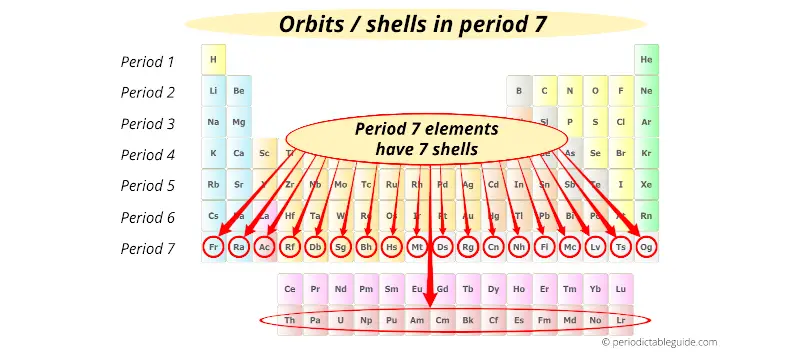
(Note: Lanthanides and Actinides are the part of period 6 and Period 7 respectively, but they are placed at the bottom of the Periodic table in order not to distort the shape of Periodic Table)
Do elements in the same period have similar properties?
NO (for main group elements)
The elements in the same Period do not have the same properties.
Reason?
Let’s take elements of period 2.
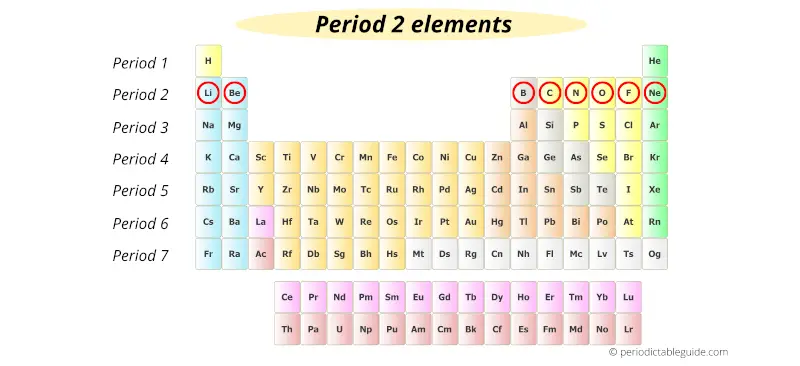
As we move from left to right across a period, the number of orbits remains the same.
But one more electron is successive added in the outermost orbit of each next element of the same period.
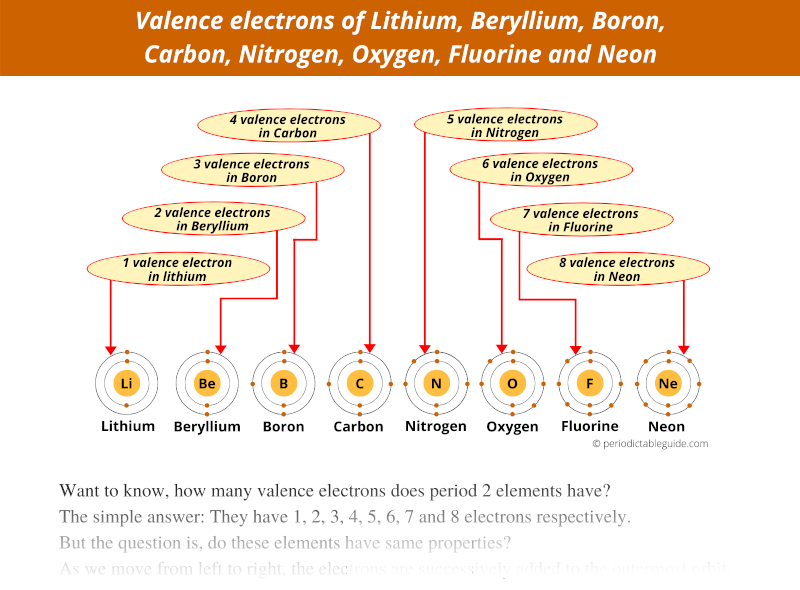
Because of this reason, their chemical properties differ.
The first element of Period 2 is Lithium (Li) which has 1 electron in outermost orbit.
Then second is beryllium (Be) which has 2 electrons in outermost orbit.
Similarly,
Boron has 3 valence electrons,
Carbon has 4 valence electrons,
Nitrogen has 5 valence electrons,
Oxygen has 6 valence electrons,
Fluorine has 7 valence electrons and
Neon has 8 valence electrons.
Thus, these elements of the same period have different valence electrons, which results in different chemical properties.
Hence, the elements of the same period do not show similar properties.
Also, you might be knowing that the elements on the left side of the Periodic table show metallic nature, while elements on the right side show non metallic nature.
Read the awesome explanation on: Metallic and Nonmetallic character trend in Periodic table.
Also visit: What does the group number tell you?
Exceptions
Mostly, the elements of the same period show different characteristics.
But for d-block elements of the same period, they show somewhat similarities in their properties.
And the f-block elements lying in the same period even show more similarities in the physical and chemical properties.
So this is it for this article.
I hope you have got your concepts clear through this article.
If you have any queries, leave a comment below.
Also let me know, has this article helped you or not?
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Suggested Important topics for you:
- Periodic table of elements (Detailed guide + HD image)
- How are the elements arranged in the modern periodic table?
- Types of metals on Periodic table
- List of elements of Periodic table
- Metals on the periodic table
- Modern periodic table with atomic mass and atomic number
- Detailed Periodic table with electron configuration
- Periodic table with electron shells
- All Periodic trends in periodic table
