The First Periodic table was arranged in the increasing order of the atomic masses of elements.
This was the first and very important arrangement created by Dmitri Mendeleev in the year 1869.
Many chemists considered this as a very important classification.

I know you can’t read anything from the above image.
But this is the real picture of the first Periodic table which was given by Dmitri Mendeleev in the year 1869.
Well, here I’ll show you how the first Periodic table was arranged.
I’ll explain this to you with a story so that you won’t get bored.
So let’s finish this very quickly.
How was the First Periodic table Arranged?
Let’s go back to the year 1869.
During the year 1869, only 63 elements were known.
Many attempts were made to classify the elements before 1869. (See the History of Periodic table for the scientists who contributed in the development of Periodic table.)
But these attempts were not upto the mark.
Now during the year 1869, Mendeleev was finding the relation between atomic mass of elements and their physical and chemical properties.
Mendeleev found the chemical properties of known elements through their chemical reaction with hydrogen and oxygen.

But the question is;
Why hydrogen and oxygen? Why not any other gas?
Because hydrogen and oxygen are the gases which react with almost all the elements.
Let me give you a few examples.
Example 1 [Sodium (Na)]
Sodium reacts with hydrogen to form sodium hydride (NaH) and it reacts with oxygen to form sodium oxide (Na2O).
Example 2 [Potassium (K)]
Potassium reacts with hydrogen and oxygen to form KH and K2O.
From the above two examples we can easily say that both sodium and potassium form similar compounds having formulae RH and R2O.
Hence they have similar chemical properties and they can be grouped together.
So according to Mendeleev, these elements should lie in the same column in his table.
Mendeleev prepared the cards of all the 63 known elements with their properties written on them.
He started arranging these elements in the increasing order of their atomic masses from left to right in a row.
If the property of the elements were similar, then he used to place it in the same column. And if the property of elements didn’t match, then he placed it in the row.
In this way, Mendeleev prepared his first Periodic table in 1869.
The modified version of Mendeleev’s Periodic table is as shown below.
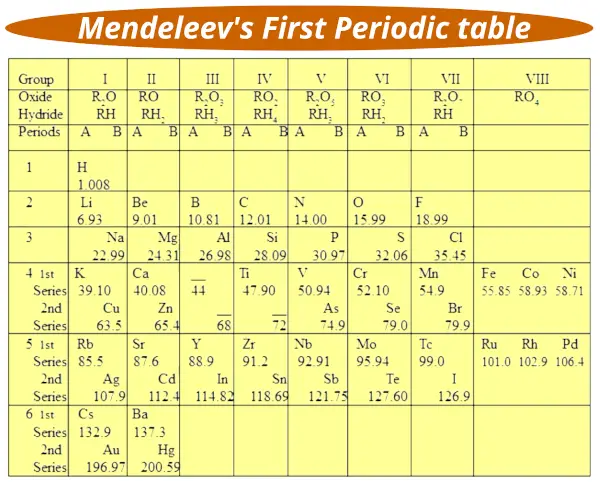
The important thing to note down here is that the elements in the above table are arranged in the increasing order of their Atomic Masses.
The vertical columns are called the groups and the horizontal rows are called the periods.
The vertical columns are again divided into two sections A and B.
There are 6 horizontal rows called periods, in which 4th, 5th and 6th periods are again subdivided into two series named “First series” and “Second series”.
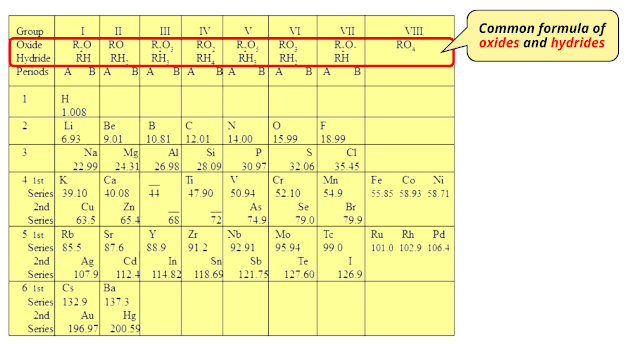
Before a few seconds I told you that Mendeleev arranged these elements on the basis of hydrides and oxides.
So in the above image you can see that formula of oxides and hydrides mentioned for each group in Mendeleev’s periodic table.
Gaps in Mendeleev’s first periodic table
Now I’ll show you what genius work Mendeleev did while preparing his table.
Have you noticed three gaps in Mendeleev’s periodic table?
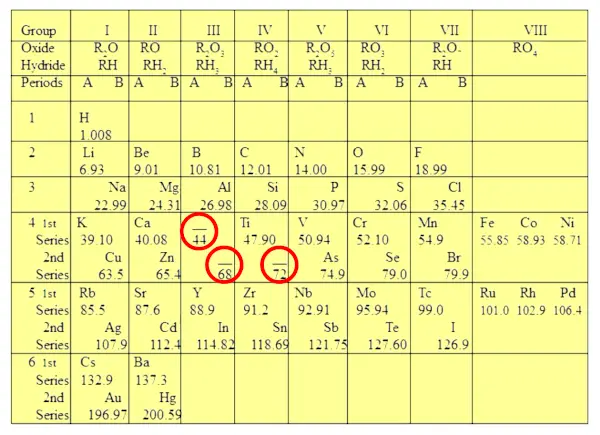
Mendeleev didn’t try to fit all the elements forcefully in his table. But instead of that, he left the gaps for the elements which were not showing the similar properties.
He predicted that those three elements were not discovered and he named those three elements as eka-boron, eka-aluminum and eka-silicon. [1]
He also predicted the properties of these three elements and also stated that they will be discovered in future.
And guess what !!
His prediction was absolutely true.
After few years, these 3 elements were discovered as;
And the main thing is that the properties of these three elements were almost similar to the properties that Mendeleev had already predicted.
This was his greatest achievement. That is why Mendeleev is also known as Father of Periodic table.
Exceptions in arrangement of Mendeleev’s periodic table
He placed some elements with greater atomic mass before the elements with lower atomic mass.
But the question is;
Why he did so?
The simple answer: Mendeleev placed some elements with higher atomic mass before lower ones, so that he can group the elements with similar properties together.
Let me give you an example from his table.
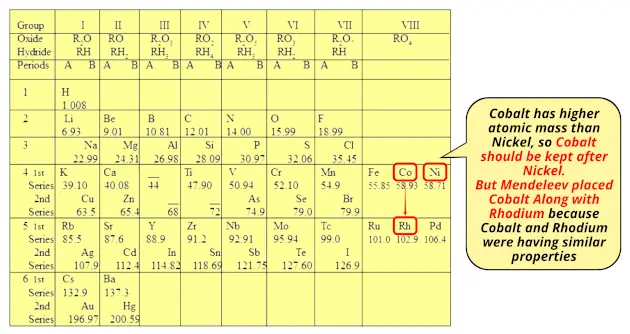
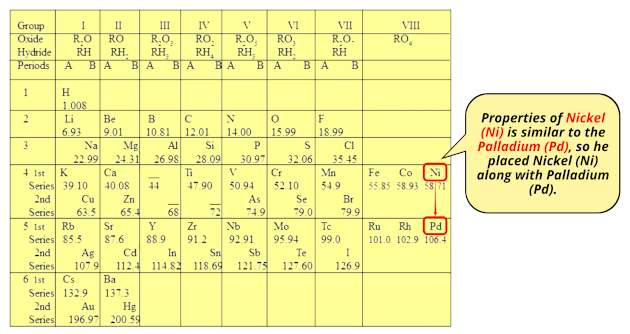
You can see that cobalt element has higher atomic mass than nickel.
So if we follow the increasing order of atomic mass, then Cobalt (Co) should be kept after nickel (Ni).
But Mendeleev placed Cobalt (Co) along with Rhodium (Rh) because Cobalt (Co) and Rhodium (Rh) were having similar properties.
Also the properties of Nickel (Ni) is similar to the Palladium element (Pd), so he placed Nickel (Ni) along with Palladium (Pd).
Tellurium (Te) and Iodine (I) also show the exceptions. Atomic mass of tellurium is higher than that of iodine. So tellurium should be kept after iodine.
But it is not so in Mendeleev’s periodic table.
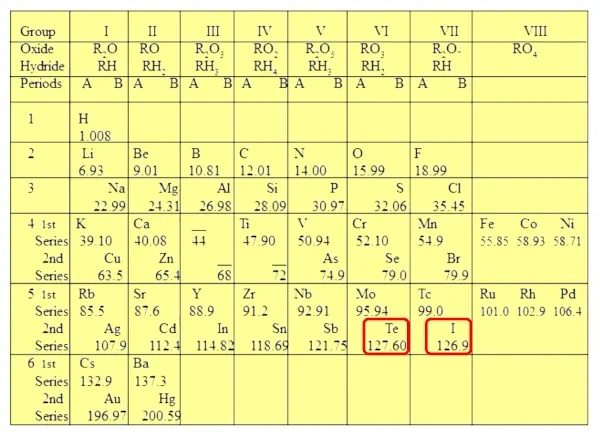
So Mendeleev’s periodic table had few exceptions in which the increasing order of the atomic mass was not followed.
What about the arrangement of Noble gases?
During Mendeleev’s time, the noble gases were not discovered.
But do you know why noble gases were not discovered during that time?
Answer: Noble gases are chemically inert. They do not react with any element. So they were not found with any compounds existing in nature.
Also the noble gases are present in very few amounts in the atmosphere.
Hence they were not discovered till 1869 during the time of Mendeleev.
Now what about the position of Noble gases in Mendeleev’s table?
In Mendeleev’s table, noble gases were not present.
But they can be placed in the separate column without disturbing the position of any elements.
This was also the great achievement of Mendeleev’s periodic table.
Final words
Mendeleev’s first periodic table published in 1869 was a huge success for the further development of Periodic table.
The elements in this First Periodic table were arranged in the increasing order of their atomic masses, and there were only 63 elements in his table.
The genius work which Mendeleev did was to leave empty gaps for the three elements whose properties were not matching.
Later on, his prediction was proved completely true when those three elements were discovered.
The elements lying in the same column were having similar properties.
The Noble gases were not discovered during Mendeleev’s time, so they were not included in his table.
I hope you have clearly understood the arrangement of elements in Mendeleev’s First Periodic table.
Let me know in the comments below, whether this article helped you or not?
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Image reference:
Dmitri Mendeleev: Via wikimedia commons.
