Here is a SUPER easy Explanation + Comparison on Mendeleev and Moseley Periodic table.
In fact, I will also show you the old age original picture of Periodic systems which was published by Mendeleev in the year 1869.
So let’s finish this very quickly.
Mendeleev’s Periodic System

I know you can’t read anything from this picture, but this is the original arrangement of elements which was done by Dmitri Mendeleev in 1869.
Let me show you a clear picture.
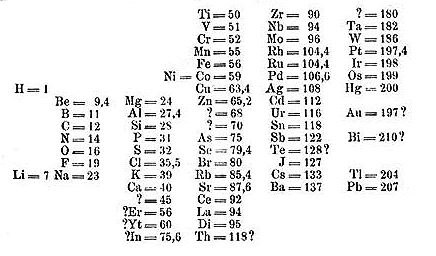
In Mendeleev’s Periodic table, the elements were arranged in the increasing order of their atomic masses.
Only 63 elements were discovered during Mendeleev’s time, so he made the arrangement of those 63 elements in his table (which is shown above). [1]
Moseley’s Periodic table
Moseley’s Periodic table was developed by Henry Gwyn Jeffreys Moseley in the year 1914.
During his time, the atomic structure was known, so he had a clear idea about the protons, neutrons and electrons.
He found that the protons are the unique identity for each and every element, and the number of protons (or atomic number) decides the chemical properties of elements.
And hence he arranged the known elements in the increasing order of their atomic number.
Moseley’s Periodic table gave us the clear concept for the arrangement of elements.
And because of his valuable efforts, today we have a complete periodic table with 118 elements without any limitations in arrangement.
Difference between Mendeleev and Moseley Periodic table
| Mendeleev’s Periodic table | Moseley’s Periodic table |
|---|---|
| 1). Arrangement of elements is based on atomic mass. | 1). Arrangement of elements is based on atomic number. |
| Explanation of 1st point The elements in the Mendeleev’s Periodic table were arranged in the increasing order of their atomic mass. While in the Moseley’s Periodic table, the elements were arranged in the increasing order of their atomic number. | |
| 2). Published in the year 1869. | 2). Published in the year 1914. |
| Explanation of 2nd point Mendeleev’s Periodic table and his observation on the Periodic system was presented to the Russian chemical society in the year 1869. While Moseley’s Periodic table was published later in the year 1914. | |
| 3). Only 63 elements were present in Mendeleev’s Periodic table in 1869. | 3). Around 92 elements were present in Moseley’s Periodic table in 1914. |
| Explanation of 3rd point There were only 63 elements discovered during the time of Mendeleev (1869). So his Periodic table contains only 63 elements. During the time of Moseley (1914), there were around 92 elements discovered. So the Periodic table presented by Moseley had 92 elements. | |
| 4). It has 8 groups & 6 periods. | 4). It has 18 groups & 7 periods. |
| Explanation of 4th point Actually the originally published Mendeleev’s Periodic table had no rows and columns (see below image).  But later on, for better understanding, the modified version was presented which contains 8 columns (groups) and 6 rows (periods). 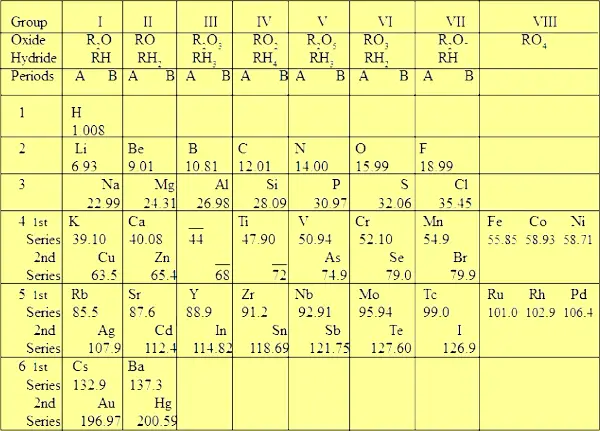 Out of the 6 periods, the 4th, 5th and 6th periods (rows) are again subdivided into two series named “first series” and “second series”. That’s why you can see two elements in a single block (see above image). While in the Moseley’s Periodic table, there are 18 groups and 7 periods. There is nothing like subdivisions in Moseley’s Periodic table. | |
| 5). Mendeleev left 3 gaps for the undiscovered elements. | 5). There were 7 gaps in Moseley’s Periodic table. |
| Explanation of 5th point In 1869, when Mendeleev published a Periodic table, there were only 63 elements discovered at that time. While arranging these known elements, he left 3 gaps for those elements whose properties were not matching with the previous ones. So he didn’t place those elements in the same column. Hence, he left 3 gaps in his Periodic table. He named those elements as eka-boron, eka-aluminium and eka-silicon. Later on, these elements were discovered and today we recognize those elements as scandium, gallium and germanium respectively. Similarly during the time of Moseley, in 1914, there were few undiscovered elements too. Moseley noticed the 3 missing elements between hydrogen (1) and gold (79). So he left gaps for those elements. These elements which were discovered later have the atomic number 43, 61 and 75. Additional four elements which Moseley found to be missing were 72, 85, 87 and 91. So there were total 7 elements missing in Moseley’s Periodic table during 1914. (source) | |
| 6). Classification was based on hydrides and oxides formed by the chemical reaction. | 6). Classification was based on electron configuration. |
| Explanation of 6th point The classification of elements in the Mendeleev’s periodic table was done on the basis of compounds like hydrides and oxides which were formed by the chemical reaction. If the elements were having similar hydrides and oxides, they were placed in the same group by Mendeleev. While in Moseley’s Periodic table, the classification was done on the basis of electron configuration of the elements. Elements with similar outer electron configuration were placed in the same group. | |
| 7). Mendeleev did not explain why elements of same groups have similar chemical properties, and elements of same periods have different properties. | 7). Moseley explained the reason behind the similar properties of elements in groups, and the reason was electron configuration. |
| Explanation of 7th point Concept: Valence electrons are the number of electrons present in the outermost orbit of an atom. Mendeleev’s Periodic table did not describe the reason why elements of the same group have similar chemical properties and why elements of the same period have different chemical properties. This is because the properties of elements depend on the valence electrons, but the atomic structure was not known during Mendeleev’s time. While, during Moseley’s time, the atomic structure was discovered and the electronic configuration of elements were known to the chemists. So based on this, he described the reason that elements lying in the group have similar electron configuration, so they have similar chemical properties. And elements of period have different electron configuration, hence they have different chemical properties. | |
| 8). Noble gases are not included in Mendeleev’s periodic table. | 8). Noble gases are included in the Moseley Periodic table (in group 18). |
| Explanation of 8th point The Noble gases are the inert gases. They do not react with any other elements. Hence it is very difficult to find Noble gases in the compound form. Also the quantity of Noble gases on the earth is very very less. So Noble gases were not discovered during Mendeleev’s time. And as they were not discovered, we do not find any Noble gases in Mendeleev’s table. While during Moseley’s time, the Noble gases were discovered. Hence he included them in separate group 18 on his table. | |
| 9). No positions for isotopes in Mendeleev’s table. | 9). The isotopes have same position along with the neutral atom. |
| Explanation of 9th point Isotopes are those variants of chemical elements which have the same number of protons and electrons, but different number of neutrons. Because of this reason, isotopes have different atomic mass as compared to the neutral atom. Mendeleev did not mention any positions for the isotopes. While in Moseley’s Periodic table, the isotopes were given the same position as that of the neutral atom. Because the Moseley’s Periodic table was arranged on the basis of atomic number (and atomic number is nothing but number of protons). In other words, isotopes have the same atomic number. | |
| 10). Some similar elements were placed in different groups while some dissimilar elements were placed in same group. | 10). There are no such misplaced elements in Moseley’s Periodic table. |
| Explanation of 10th point In Mendeleev’s periodic table, to maintain the increasing order of atomic mass, some elements which possess similar chemical properties were placed in different groups, while some dissimilar elements were placed in the same group. While in Moseley’s Periodic table, there are no such misplaced elements. In Moseley’s Periodic table, the arrangement of elements is done on the basis of atomic number. And the atomic number is a unique number for each element. No elements can have two different atomic numbers. That’s why there are no such misplaced elements. | |
| 11). The properties of elements were not repeating after regular intervals. | 11). The properties of elements were repeating after regular intervals. |
| Explanation of 11th point In Mendeleev’s periodic table, the elements are arranged on the basis of atomic mass. And the fact is that the chemical properties of elements do not depend on atomic mass, but it depends on atomic number. So Mendeleev’s periodic table arrangement do not show repetitive properties in elements. While in Moseley’s Periodic table, the elements are arranged on the basis of atomic number, and atomic number (actually valence electrons) only decides the chemical properties. Hence Moseley’s Periodic table shows repetitive properties after regular intervals of 2, 8, 8, 18, 18,… | |
| 12). Separate positions for metals and Nonmetals were not present. | 12). Metals, Nonmetals and semimetals have separate positions. |
| Explanation of 12th point In Mendeleev’s periodic table, there are no separate positions for metals and nonmetals. But they are all found together. While in Moseley’s Periodic table, they are at specific positions. The metals are on the left, nonmetals are at the right and semimetals are located in between them. See the: Periodic table with Metals, Nonmetals and Metalloids. | |
| 13). Some elements with higher atomic mass were placed before the elements with lower atomic mass. | 13). All the elements were arranged in the increasing order of their atomic number. |
| Explanation of 13th point Mendeleev made the arrangement of elements on the basis of increasing atomic masses. But to maintain the similarities in the properties, some elements with higher atomic mass were placed before the elements with lower atomic mass. While in Moseley’s Periodic table, all the elements were arranged in increasing order of atomic number. | |
| 14). Group number and period number of elements cannot be predicted from Mendeleev’s table. | 14). Group number and period number of any element can be perfectly found from Moseley’s Periodic table |
| Explanation of 14th point Mendeleev’s table is based on atomic mass, but Moseley’s table is based on atomic number. From atomic number, we can easily find the electron configuration and finally we can find the position of elements in periodic table. | |
| 15). Transition elements were found along with the other elements in Mendeleev’s Periodic table. | 15). Transition elements were classified in separate block (d block) in Moseley’s Periodic table. |
| Explanation of 15th point In Mendeleev’s periodic table, there are no block wise classification because there was no knowledge of electronic configuration and orbitals. Hence, there is no block wise classification of transition elements on Mendeleev’s periodic table. While in Moseley’s Periodic table, the knowledge of orbitals was much developed and hence we can see s p d and f block on the Moseley’s Periodic table. Transition elements have the valence electrons in d-orbitals, hence they are classified into d-block on Moseley’s Periodic table. | |
Summary
I hope you have clearly understood the Mendeleev and Moseley Periodic table.
You can also remember the difference between Mendeleev and Moseley Periodic table from this single image given below.
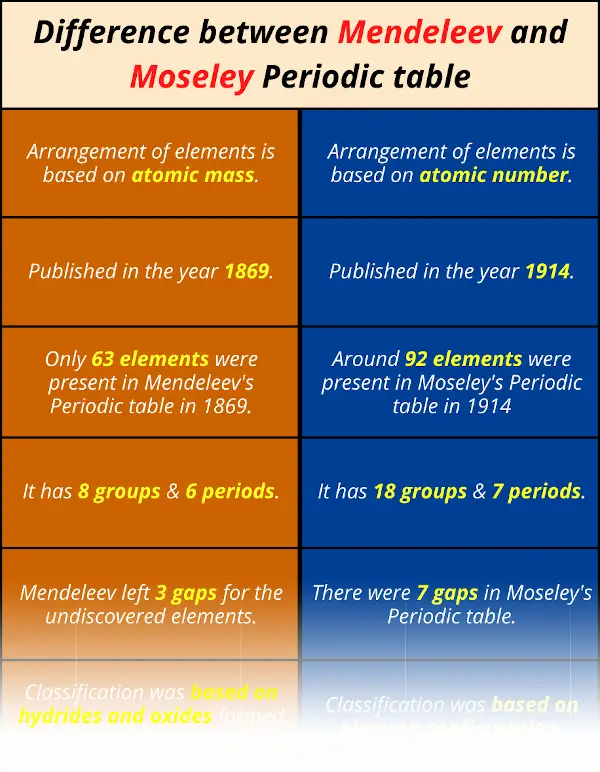
If I will give you all the differences in this single image, then this image will be very long.
Hence, refer the above table for all the differences (along with their explanations.)
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
