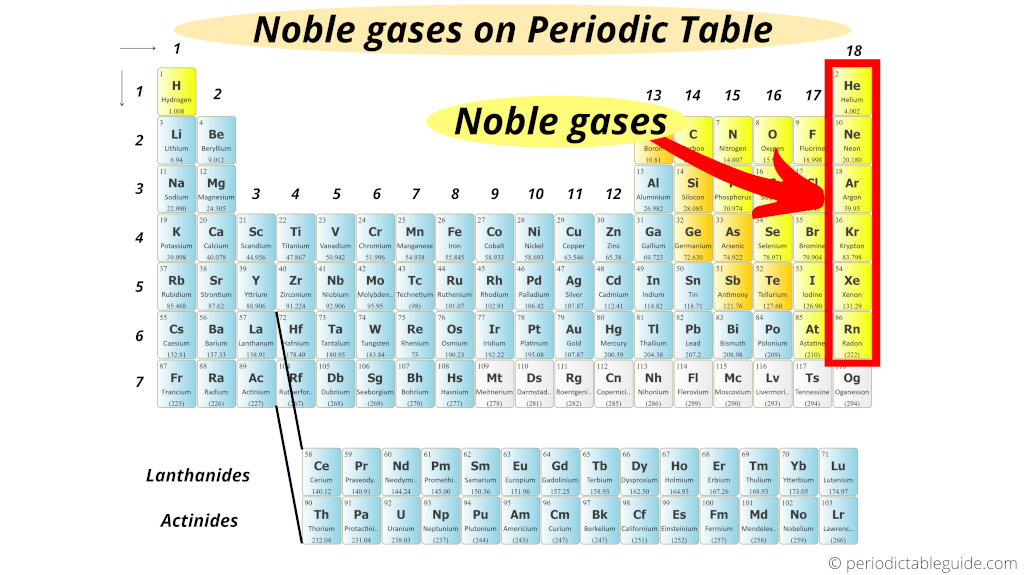
Noble gases are located in the right most group of the Periodic table (i.e group 18).
This above image exactly shows you where are noble gases located on the Periodic table.
There are total 6 known noble gases on the Periodic table.
Oganesson (Og) is predicted to have similar properties like Noble gases, but it also shows some reactivity unlike noble gases.
It is a laboratory made synthetic element and it is still under research work.
But wait…
There are lot more things you need to know about the noble gases like;
- What exactly are the noble gases? And why are they called so?
- List of Noble gases
- Electronic configuration of noble gases
- What do noble gases have in common?
- Uses of Noble gases in daily life
- Noble gases facts
- Where are noble gases found in nature?
Let’s finish this quickly.
What are noble gases? And why are they called so?
Noble gases are the six chemical elements present in the group 18 of the Periodic table.
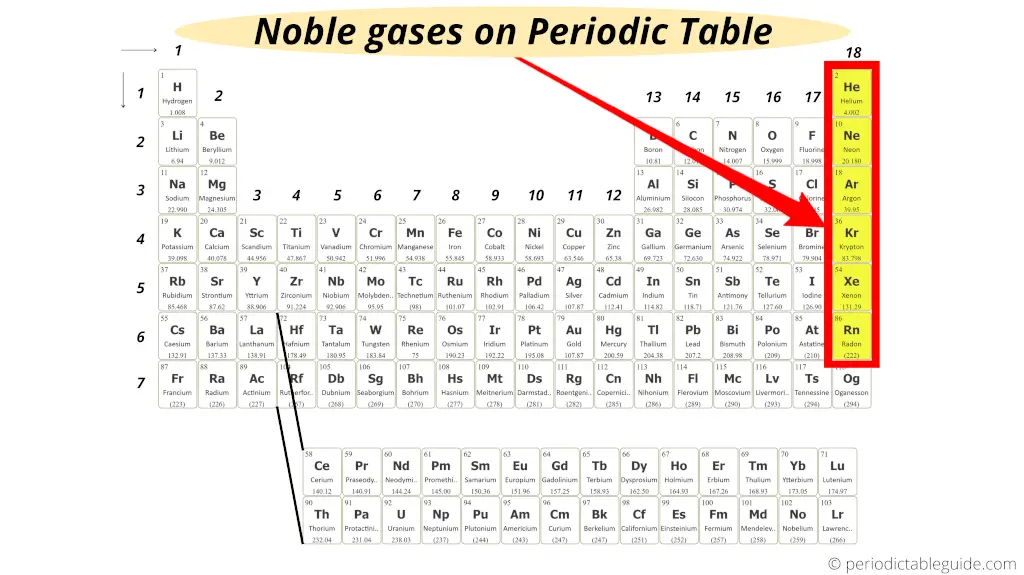
They are Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe) and Radon (Rn).
The outermost orbit of these noble gas elements are completely filled with electrons.
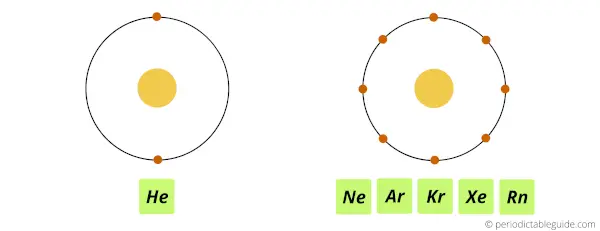
Helium element has 2 electrons in its outermost orbit, while the rest of the noble gases (Ne, Ar, Kr, Xe, Rn) have 8 electrons in their outermost orbit.
This completely filled outermost shell makes them very stable.
So these elements of group 18 are the noble gases.
Why are they called noble gases/inert gases?
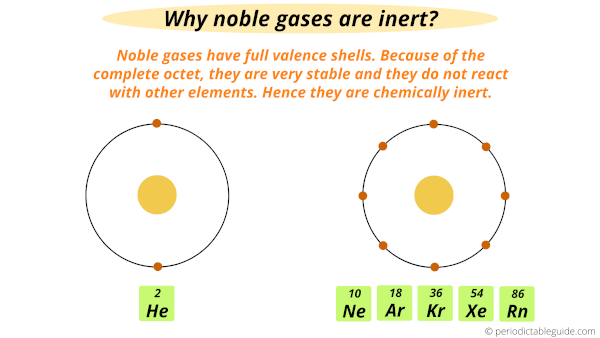
Group 18 elements are called noble gases because they normally do not react with any other elements.
All these noble gases have full valence shells and this makes them a very stable element.
In fact, they are so stable that in the past chemists thought that they could not react with any other elements (means they are chemically inert).
This is why they were called inert gases (or unreactive gases).
However, later on chemists found that few of these noble gases can react with some elements to form compounds.
For example:
Hence, group 18 elements are called Noble gases.
List of Noble gases
The list of Noble gases with atomic number, symbol and name of element is given below.
| Atomic number | Symbol | Name of element |
| 2 | He | Helium |
| 10 | Ne | Neon |
| 18 | Ar | Argon |
| 36 | Kr | Krypton |
| 54 | Xe | Xenon |
| 86 | Rn | Radon |
Electronic configuration of noble gases
The general outer electron configuration of Noble gases is ns2 np6 where n is the number of outer orbit or period number in which the element lies.
Helium is an exception whose electron configuration is 1s2.
The shell electron arrangement and electronic configuration of Noble gases is shown below:
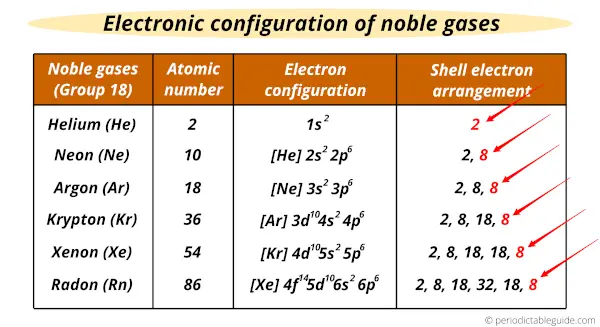
Here you can see that the outermost orbit has 8 electrons (Except helium. Helium has 2 valence electrons) which forms a stable octet.
This indicates that all the noble gas elements are stable and mostly these group 18 elements are chemically inert.
What do noble gases have in common?
Short answer:
- They have the same number of valence electrons.
- They are colourless gas.
- They are odourless gas.
- They are monoatomic.
Let me give you a short description of the above points.
The noble gases have the common number of valence electrons (i.e 8 valence electrons)
In other words, the outermost shell of all the noble gases have the same number of electrons (i.e 8).
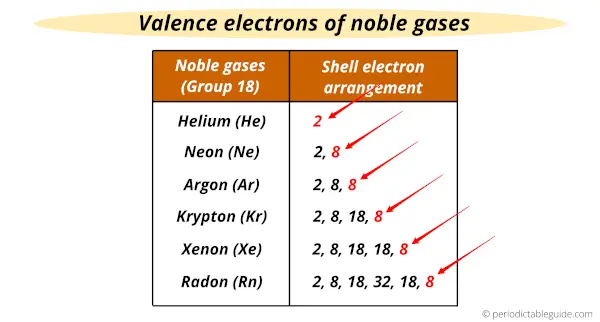
Helium (He) is an exception which has only 2 valence electrons.
Also, the noble gases are colourless gas, that means we can’t see it.
The noble gases are odourless gases, that means they have no smell.
Noble gases exist as a monoatomic gas in nature. That means they have a single atom.
(Side note: Monoatomic gas has a single atom. For example: He, Ne, Ar, etc… While diatomic have 2 atoms, for example: H2, O2, F2, etc.)
Uses of Noble gases in daily life
The uses of Noble gases are mentioned below.
#1 Helium: Air-ships and balloons
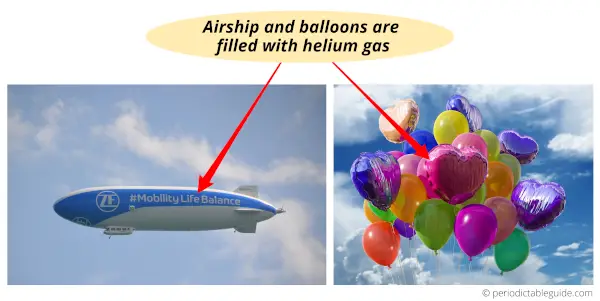
Helium is lighter than air and so it is used in air-ships and air balloons. As helium is lighter than air, the air-ships and balloons float in the air.
#2 Neon: Neon signs

Neon gas is used in sign boards.
When high voltage electricity is passed through the neon gas, it glows. And thus the neon gas is used in sign boards.
#3 Argon: Filament bulb

The filament of the bulb burns at a higher temperature when electric current is passed through it.
Argon is filled in this filament bulb. Being a chemically inert gas, argon prevents the burning of filament and also reduces its evaporation.
#4 Krypton & Xenon: Fluorescent bulb

Krypton or Xenon gases are used in the flash bulbs, lazers and fluorescent bulbs.
#5 Radon: Radiotherapy
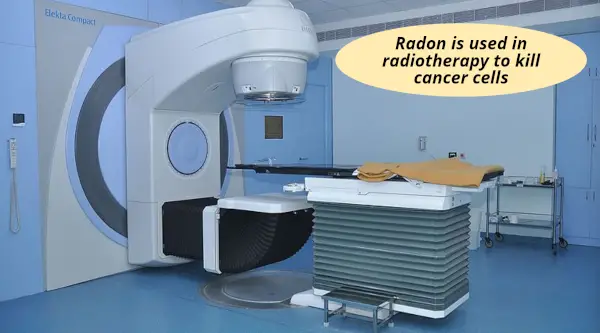
Radon is chemically inert gas, but it is radioactive in nature.
Thus, radon is used in radiotherapy (or cancer therapy) to kill the cancer cells.
Noble gases facts
The facts of Noble gases are mentioned below.
- Helium is the second most abundant element found in the Universe and it makes roughly up to 24% of it. (Note: Hydrogen is first and it is roughly 74% of all the elements in the universe)
- Helium is always floating out of the earth’s atmosphere. On the earth, helium is obtained from the decay of radioactive elements like uranium and thorium.
- Neon is about two-thirds as dense as that of air.
- Neon is the fifth most abundant element found in the universe followed behind hydrogen, helium, oxygen and carbon.
- Argon is the first noble gas to be discovered.
- Argon is 38% denser than air, so it remains closer to earth’s surface when it is used.
- Quantity of Xenon is very less in the earth’s atmosphere. It is around 0.087 parts per million.
- There are more than 40 radioactive isotopes of xenon.
- Radon was discovered by scientist Friedrich Ernst in 1900 and it was named radium emanation. Later on it was named Radon by IUPAC in 1923.
Where are noble gases found in nature?
The noble gases are found in nature in the following manner.
- Helium is present in abundance in all the stars (including sun). On the earth helium is very rare and it is obtained from the decay of radioactive elements like uranium and thorium.
- The concentration of neon in the atmosphere is about 1 part in 55,000 or 18.2 ppm by volume or 1 part in 79,000 of air by mass.
- Neon is created as a byproduct of the liquefaction of air.
- Argon makes up 1.2% of the earth’s atmosphere.
- NASA’s probe has found that Argon is present in the atmosphere of planet Mercury. Argon is also found on Saturn’s moon Titan.
- Krypton is found in earth’s atmosphere at about 1 part per million.
- Krypton is also found in the atmosphere of planet Mars with 0.3 parts per million quantity.
- Concentrations of Kr-85 are 30% higher at the North pole than that are at the South Pole.
- Xenon is found in Mars’s atmosphere at about 0.08 parts per million.
- Jupiter has exceptionally high amount of Xenon, almost 3 times that of the sun.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Summary
So in the beginning of the article, I showed you a single image which shows you where are noble gases located on the Periodic table.
The noble gases are located in the right most group (i.e group 18) of the Periodic table.
Then we discussed;
- What exactly are the noble gases? And why are they called so?
- List of Noble gases
- Electronic configuration of noble gases
- What do noble gases have in common?
- Uses of Noble gases in daily life
- Noble gases facts. And finally…
- Where are noble gases found in nature?
I hope this article has helped you in solving your doubts.
Feel free to ask me in the comments, if you have any doubts.
Also let me know whether this article has helped you or not?
Suggested Important articles for you:
- Periodic table of elements (Detailed guide + HD image)
- Metals on the periodic table
- Nonmetals on the periodic table
- Metalloids on periodic table
- Halogens on periodic table
- Alkali metals on periodic table
- Alkaline earth metals on periodic table
- Transition metals on Periodic table
- Inner transition metals on periodic table
- Periodic trends in periodic table
