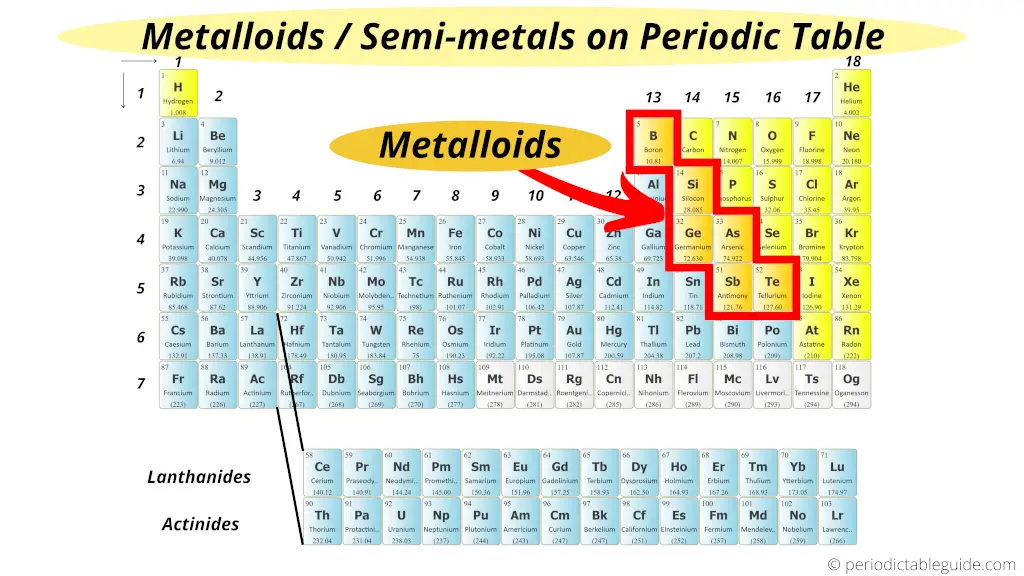
Metalloids are located between the metals and nonmetals. The orange color on the Periodic table represents metalloids.
They form a separating boundary between the metals and nonmetals.
In other words, metalloids (semimetals) are located on the right side of the post transition metals and on the left side of nonmetals (see above image).
Also we can say that metalloids are present in the diagonal region of the p block on Periodic table.
I hope you have got the answer of “Where are Metalloids located on the periodic table?”
But wait…
Because there are lot more things you need to know about the metalloids and most of the students have confusion regarding the total number of metalloids present on the Periodic table.
I have solved all those doubts in this single small article.
If you want to skip to any part of this article, just click on the below links !!!
- What exactly are the metalloids?
- How many metalloids are there in the Periodic table?
- List of metalloids on the periodic table
- Why are metalloids called semiconductors?
- What are the properties of metalloids?
What are metalloids on the periodic table?
Metalloids:
The elements that show some properties of metals as well as solid nonmetals are called metalloids.
- Metalloids look like metals, but they are not.
- Metalloids are brittle like solid nonmetals.
- Metalloids are neither conductor nor insulated.
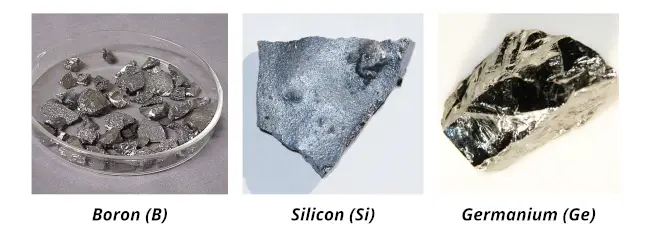
The examples of metalloids are:
Now let me explain all these things to you in a simple way.
Actually there is not a widely agreed definition of metalloids but in the literature of chemistry, the metalloids are considered as those elements which show properties in between the metals and nonmetals.
Some metalloids elements may show properties that are a mixture of metallic properties as well as nonmetallic properties.
They have few traits of metals as well as few traits of nonmetals.
They are neither completely metals nor completely nonmetals.
Hence the elements which show some properties of metals and some properties of nonmetals are known as metalloids.
How many metalloids are there in the Periodic table?
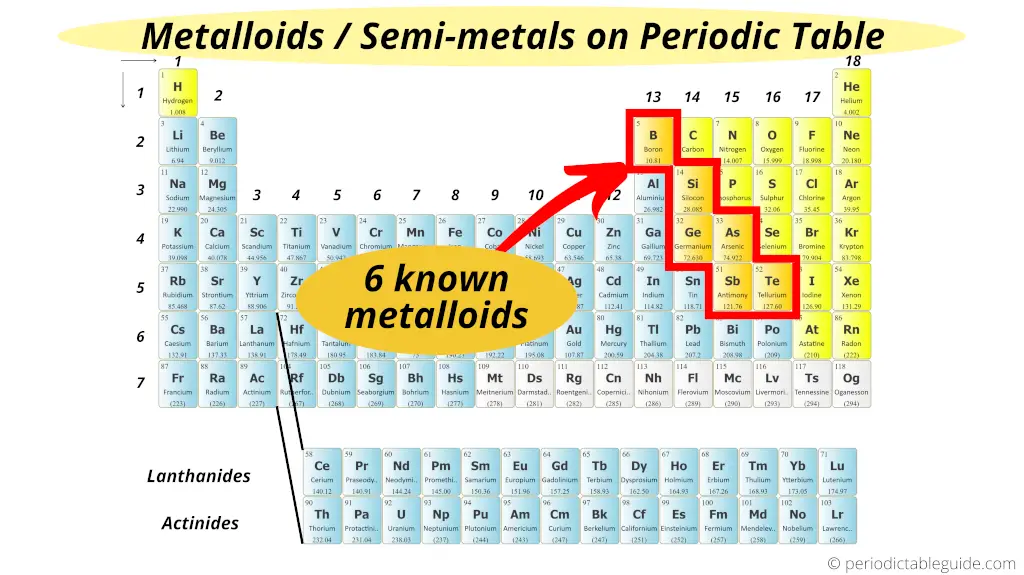
Six
Yes, there are 6 commonly known metalloids on the Periodic table.
But wait…
This is not the exact number.
Reason?
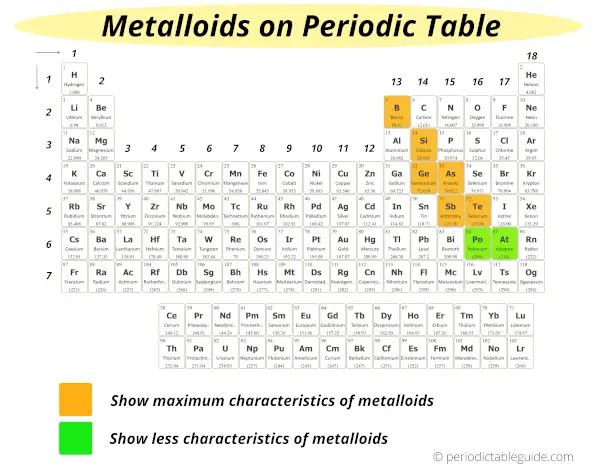
As you can see in the above image, there are six known metalloids which show maximum characteristics of the metalloids and they are represented in orange color.
These elements which shows maximum characteristics of metalloids are:
- Boron (B),
- Silicon(Si),
- Germanium(Ge),
- Arsenic(As),
- Antimony(Sb) and
- Tellurium(Te).
But the other two elements also show few characteristics of metalloids and they are represented in green color on the above Periodic table.
These two elements which show fewer characteristics of metalloids are:
Now the main questions are,
Is Polonium a metalloid?
Is Astatine a metalloid?
Should we consider Polonium and Astatine as metalloids?
Many researchers have found that Polonium shows more characteristics of metals. And Astatine shows more characteristics of nonmetals (halogens).
Thus Polonium and antimony are not included in the category of metalloids by many researchers. (Source: HRW texts)
Also this number is inexact due to lack of universally accepted definitions.
(That means in metallurgy, the researchers may define metalloids on the basis of density. In physics, they may define metalloids on the basis of physical properties. And in chemistry, the chemists are concerned with the chemical properties of metalloids.)
Also, Polonium and Astatine are synthetic elements and they have very short half life.
Hence, there are total 6 known metalloids/semimetals on the Periodic table.
Let us now see the list of metalloids/semimetals with their atomic number, symbol and name.
List of metalloids on the periodic table
Here is a complete list of metalloids on the Periodic table.
| Atomic number | Symbol | Name of element |
| 5 | B | Boron |
| 14 | Si | Silicon |
| 32 | Ge | Germanium |
| 33 | As | Arsenic |
| 51 | Sb | Antimony |
| 52 | Te | Tellurium |
Why are metalloids called semiconductors?

Metalloids are called semiconductors because they are not good conductors like metals and also they are not bad conductors like nonmetals.
They have the conductivity which is higher than nonmetals, but lower than metals.
Hence metalloids are known as semiconductors.
Also if you know about the conduction band and valence band, then read further.
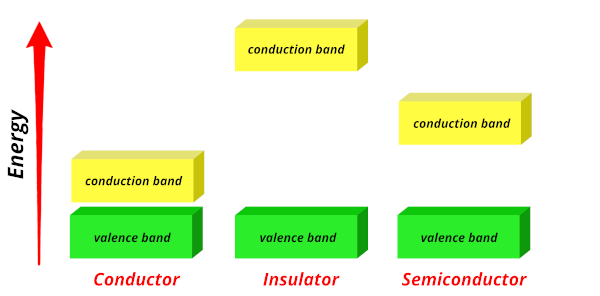
The green color shows the valence band, while yellow color shows the conduction band.
Let us understand the phenomenon in conductors, insulators and semiconductors.
Conductors:
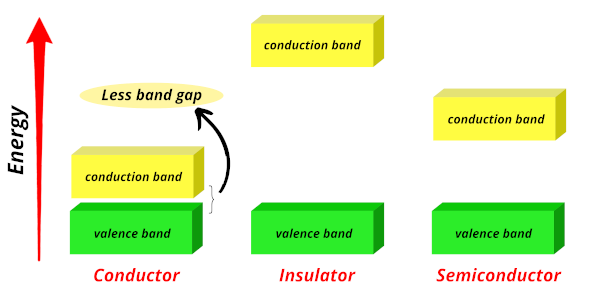
In conductors, the valence band and conduction band are very close to each other. The free electrons can easily jump from the valence band to the conduction band.
The free electrons do not require more energy to jump from valence band to conduction band.
Hence, due to more transfer of electrons, they have more conductivity.
Examples of good conductors are: Silver, copper, aluminum, etc…
Insulators:
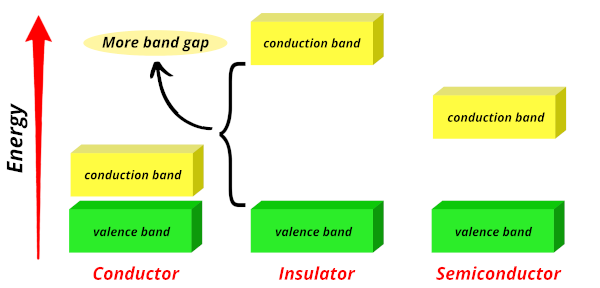
In insulators, the band gap is very large as shown in the above image.
The electrons require more energy to jump from the valence band to the conduction band.
Thus they have less conductivity. And hence they are known as poor conductors or insulators.
Semiconductors:
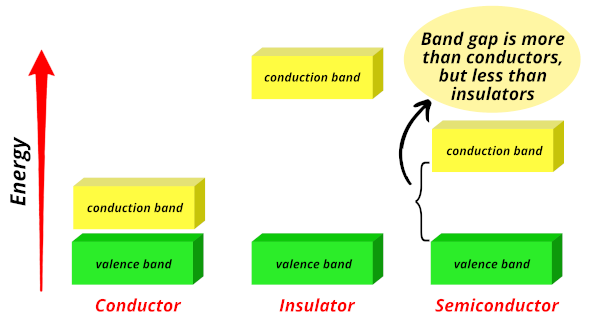
Now in semiconductors, the band gap shown in the above image is not so high as that of insulators and not so close as that of metals.
But the band gap of semiconductors is in between the two.
Hence in semiconductors, for the electrons to jump from valence band to conduction band, they require less energy than that of insulators and more energy than that of conductors.
Hence those elements whose energy band gap lies in between the metals and insulators are known as semiconductors.
Example: Silicon is a widely used semiconductor nowadays.
What are the properties of metalloids?
Let us discuss the physical properties as well as chemical properties of metalloids/semimetals.
Physical properties of metalloids
Physical properties metalloids are somewhat similar to that of metals.
- Metalloids have metallic appearance.
- They are solid at room temperature.
- They are brittle in nature.
- They are less conductive than metals and more conductivity than nonmetals.
Chemical properties of metalloids
Chemical properties of metalloids are little bit similar to that of nonmetals.
- Metalloids + Metals = Alloys (When they are mixed with metals, they forms alloys)
- Some metalloids contract when they are melted.
- Metalloids + Halogens = Compounds (metalloids elements reacts with halogens and finally compounds are formed by this chemical reaction)
- Metalloids have different metallic allotropes as well as nonmetallic allotropes.
- Metalloids have the property to form glasses on oxidation and so that are used in glass manufacturing.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Summary
So in this article, we discussed the definition of metalloids. Then we discussed the position of metalloids in the Periodic table.
Later on we saw that there are 6 metalloids on the Periodic table.
Then we discussed the complete list of all the metalloids with their atomic number, symbol and element name.
Then I gave you the reason why metalloids are called semiconductors.
And finally we discussed the physical and chemical properties of metalloids.
I hope this article “Where are Metalloids located on the Periodic table?” has helped you solve your query.
Feel free to comment your views in the comment section below.
Suggested Important articles for you:
- Periodic table (with everything you need to know) (Important)
- Metals on the periodic table
- Nonmetals on periodic table
- Halogens on periodic table
- Alkali metals on periodic table
- Alkaline earth metals on periodic table
- Noble gases on periodic table
- Transition metals on Periodic table
- Inner transition metals on periodic table
- What do elements in the same group have in common
