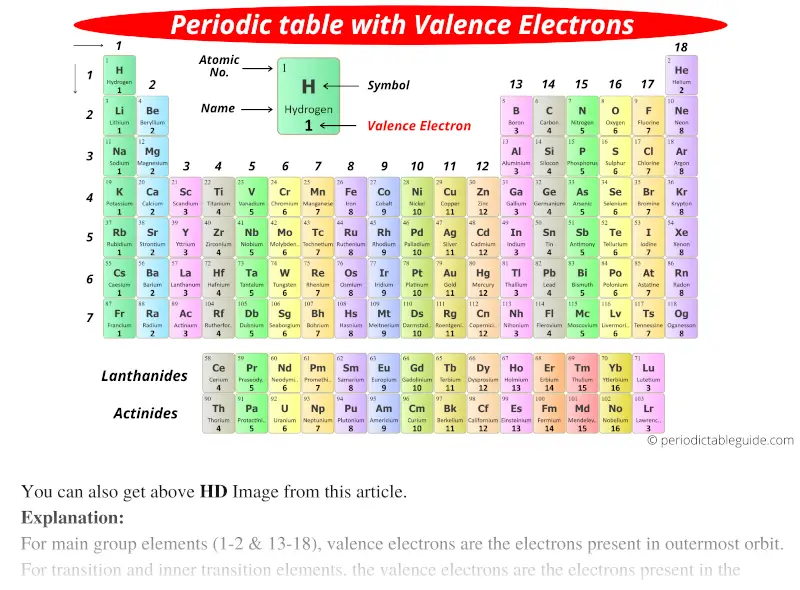
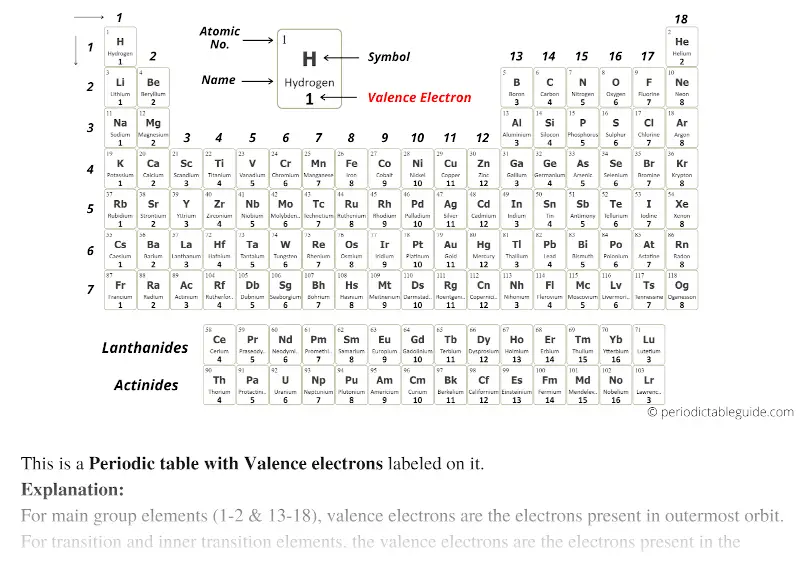
The above image clearly shows you the Periodic table with Valence Electrons labeled on it.
You can also get the printable Periodic table with valence electrons, from this article only. (Downloading link given below)
There are many more labeled Periodic Table of valence electrons given below.
But before that, just have a look at the concept of valence electrons.
Valence electrons: The electrons present in the outermost orbit of an atom are called valence electrons.
For example;
Let’s take an example of a magnesium element.
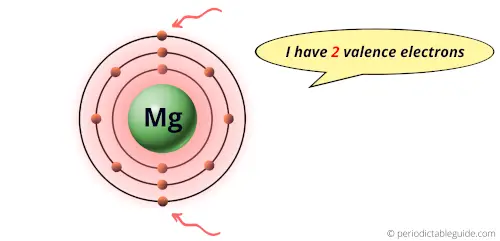
The magnesium element has 2 electrons in outermost orbit.
Hence it has 2 valence electrons.
Valence electrons can also be determined as the electrons present in the shell with highest principal quantum number (n).
For example,
The electron configuration of magnesium is 1s2 2s2 2p6 3s2.
Here, you can see that the highest principal quantum number is 3, and the total electrons in this principal quantum number is 2.
Hence, magnesium has 2 valence electrons.
Well, this suits perfectly for the main group elements (i.e group 1, 2 and group 13 to 18), but what about the transition and inner transition elements?
What about valence electrons of transition and inner transition elements?
For the transition elements and inner transition elements, the case is more complicated.
It is more difficult to find the valence electrons of transition elements as they have incompletely filled d-subshell and this d-subshell is very close to the outer s-subshell. So, the electrons of both d-subshell and s-subshell behave like valence electrons.
Hence, the transition elements (i.e d-block elements from group 3 to 12) can have more valence electrons ranging from 3 to 12. (See the above or below periodic table to see the valence electrons of transition metals).
Also the two bottom rows at the bottom of the periodic table are the inner transition elements (or f-block elements) also have the similar case.
The inner transition elements have incomplete f-subshells and they are very close to the outer s-subshell. Hence, for inner transition elements, the electrons of both f-subshells as well as s-subshell behave like valence electrons. In some inner transition metals, the electrons of incomplete d-orbitals are also considered as valence electrons.
Inner transition elements can have valence electrons ranging from 3 to 16. (See the above or below periodic table to see the valence electrons of inner transition metals).
Point to Remember: Mostly for transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
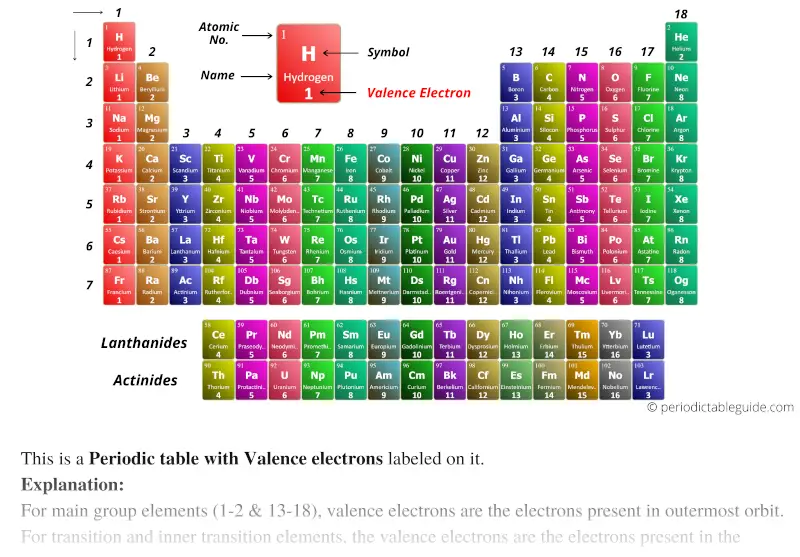
Periodic table with valence electrons (Dark colors)
Periodic table with valence electrons and blocks
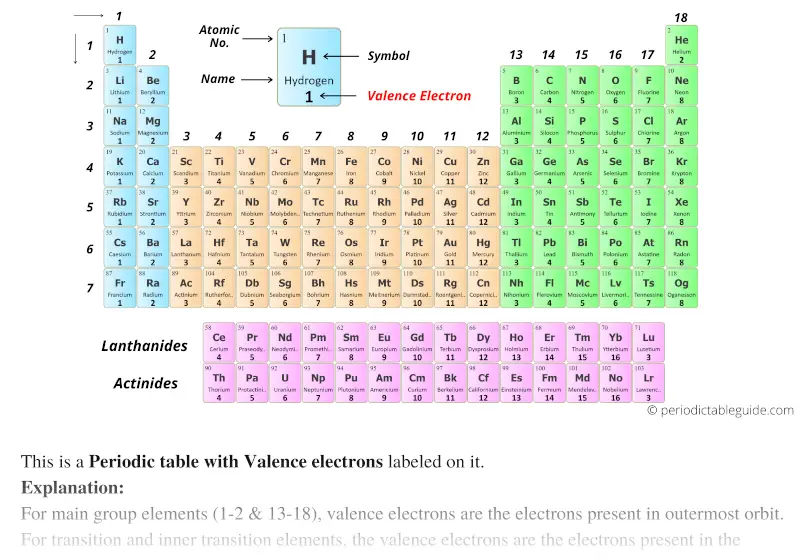
The elements in blue colour are the s-block elements.
Green coloured elements are p-block elements.
Orange coloured elements are d-block elements and purple elements are the f-block elements.
Black and White
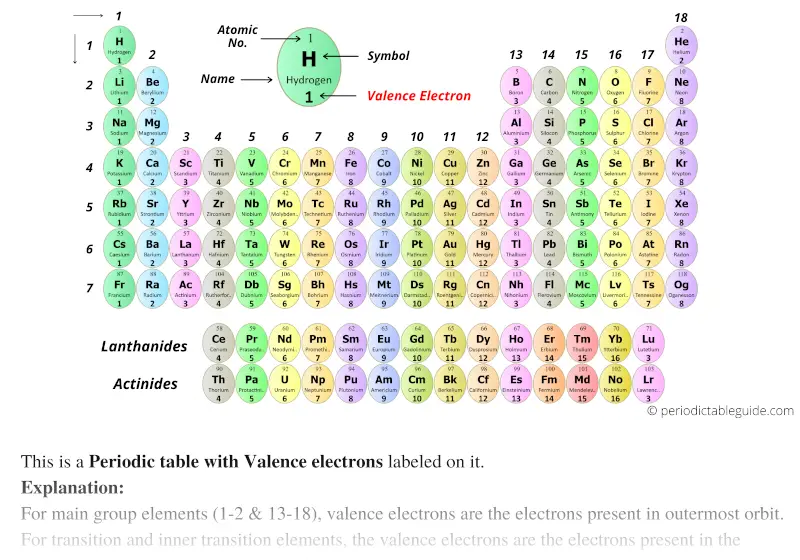
With circular tile
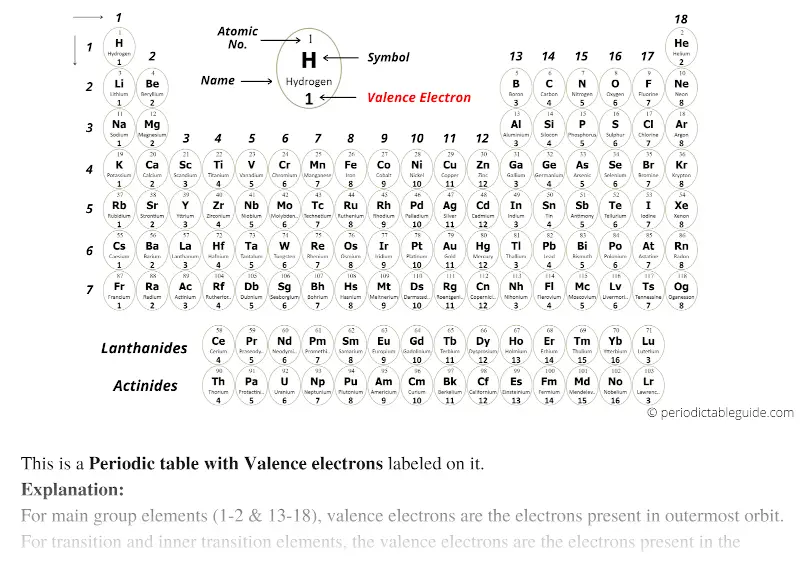
With circular tile (Black and white)
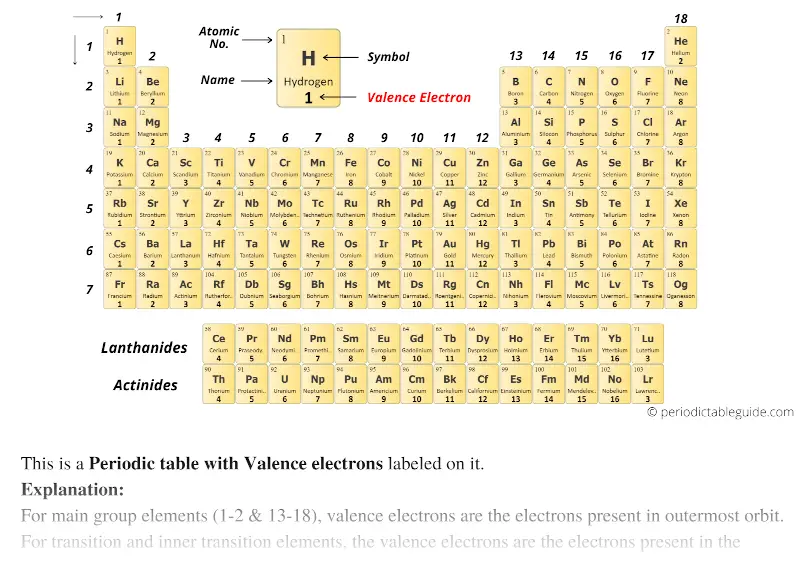
Single colored Periodic table
Valence electrons of Elements (List)
Here is the list of periodic table of elements with their valence electrons.
| Atomic number | Elements | Valence electrons |
|---|---|---|
| 1 | Hydrogen | 1 |
| 2 | Helium | 2 |
| 3 | Lithium | 1 |
| 4 | Beryllium | 2 |
| 5 | Boron | 3 |
| 6 | Carbon | 4 |
| 7 | Nitrogen | 5 |
| 8 | Oxygen | 6 |
| 9 | Fluorine | 7 |
| 10 | Neon | 8 |
| 11 | Sodium | 1 |
| 12 | Magnesium | 2 |
| 13 | Aluminum | 3 |
| 14 | Silicon | 4 |
| 15 | Phosphorus | 5 |
| 16 | Sulfur | 6 |
| 17 | Chlorine | 7 |
| 18 | Argon | 8 |
| 19 | Potassium | 1 |
| 20 | Calcium | 2 |
| . | . | . |
| . | . | . |
| . | . | . |
These are the Valence electrons of first 20 Elements.
But if you want to see the valence electrons of all the 118 elements, then visit: Valence electrons of all the elements. (Make sure you visit this, because I have mentioned the valence electrons along with the images for each element).
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
