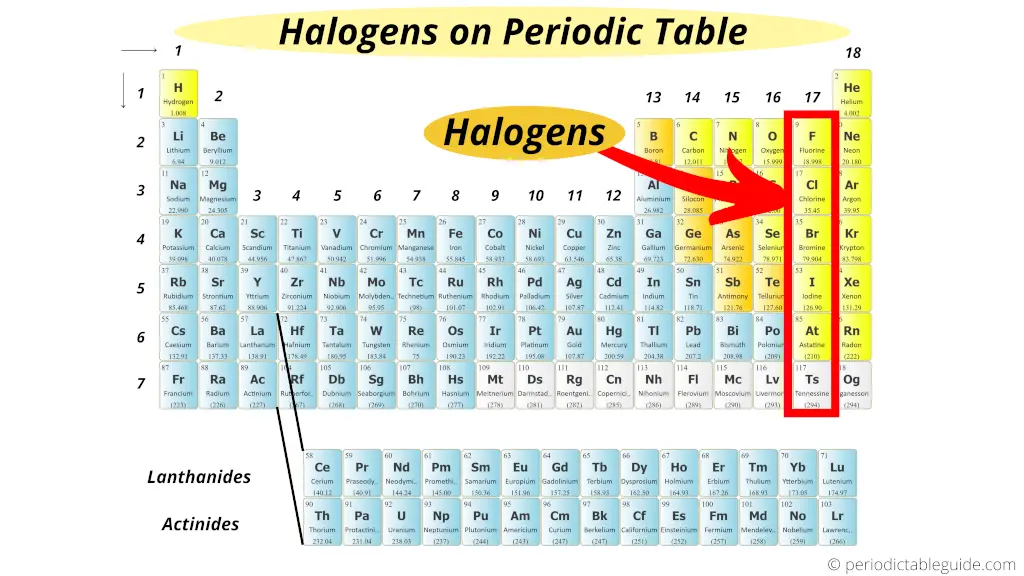
Here they are !!!
Halogens are located in the 17th group of Periodic table (exactly to the left of Noble gases).
Well, I hope you have got the exact answer for your question “Where are the halogens located on the periodic table?” from this above image.
But there are a lot more things you need to know about halogens in the Periodic table, like;
- What exactly are the halogens?
- Why group 17 elements are called halogens?
- Which is the most reactive halogen?
- List of halogens
- Is Astatine a halogen?
- Properties of halogens
So if you want to skip to any of your interested topics, just click on the above links to navigate on that topic.
Let’s get started.
What are halogens? And why group 17 elements are called halogens?
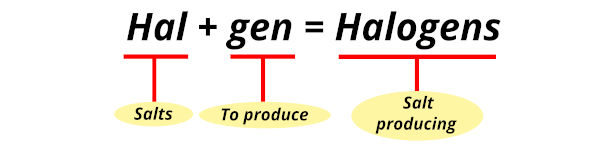
The word halogen has derived from two Greek words.
- Hal means “salts” and
- Gen means “to produce”
So halogens means salt producing.
In other words, these halogen elements form salts when they react with metals.
For example,
2Na + Cl2 ———> 2NaCl
Here chlorine (Cl) is a halogen, and when it reacts with metals (sodium, Na), it will form a salt (NaCl).
There are 5 known halogens on the Periodic table.
These halogens lies in group 17 of Periodic table and all these halogen are salt-former (means they forms salt on reacting with metals)
Hence these group 17 elements are known as halogens.
Which is the most reactive halogen?
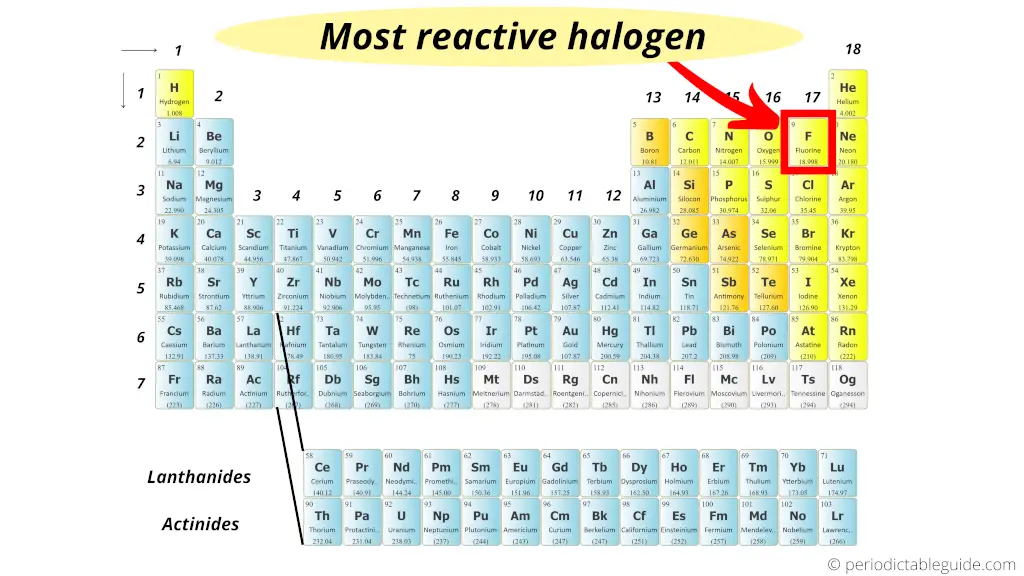
Out of all the halogens, the Fluorine (F) is a highly reactive halogen present on the Periodic table.
But do you know why is fluorine most reactive halogen?
Let me explain.
I’ll tell you the exact reason behind why fluorine is highly reactive halogen on the Periodic table.
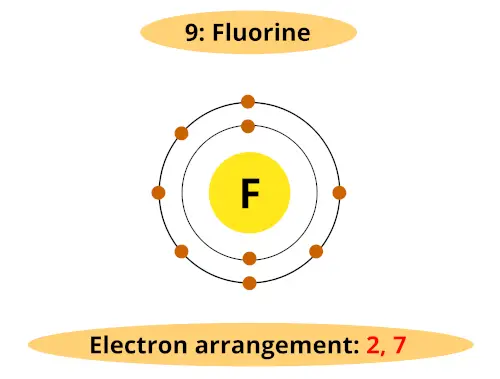
Do you know, what is the atomic number of fluorine? It’s 9, Right?
Thus it has total 2 orbits and it’s electron shell arrangement is 2, 7.
It has 7 electrons in its outermost orbit as shown in the shell diagram below.
Now you know that the atom has to complete the octet to become stable.
So here fluorine requires only 1 electron to complete the octet (7 + 1 = 8).
And it is very easy to gain one electron during a chemical reaction.
Also the atomic size of fluorine is very small. (For more information on atomic size trend, check this Periodic table guide)
And because of its smaller size and need of only one electron, it is the most electronegative element in the entire Periodic table.
Electronegativity is nothing but a tendency to attract the electron pair towards it.
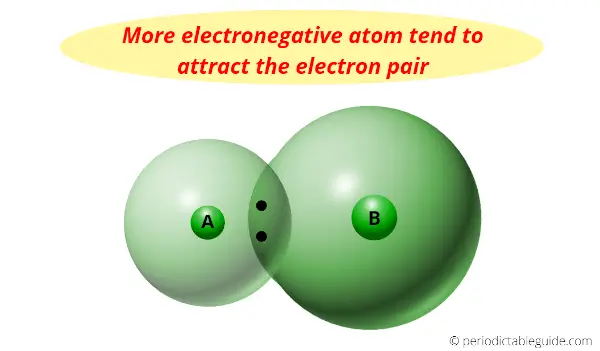
Now see, the halogens are the most reactive elements, but we know that as we move down the group, the electronegativity decreases (means the tendency to attract the electron pair decreases down the group).
In other words, Fluorine is present at the top of the halogen group and it has less atomic size plus it needs only one electron to complete the octet.
So the electronegativity of fluorine is highest (it has maximum tendency to attract the electrons pair)
And because of this, fluorine has a highest tendency to react with any other element to form a compound.
Even fluorine reacts with noble gas like xenon, and forms compounds like XeF4 (Xenon tetrafluoride) and XeF6 (Xenon hexafluoride)
Thus, fluorine element of group 17 is a highly reactive halogen on the Periodic table.
(Note: Noble gases have even smaller atomic size compared to halogens, but they have complete octet. So they do not participate in any chemical reaction. Hence noble gases are chemically inert)
List of Halogens
Here is a complete list of halogens (group 17 elements) of the Periodic table with their atomic number, symbol and name of element.
| Atomic number | Symbol | Name of elements |
| 9 | F | Fluorine |
| 17 | Cl | Chlorine |
| 35 | Br | Bromine |
| 53 | I | Iodine |
| 85 | At | Astatine |
Is Astatine (At) included in halogens?

Yes and No.
You read it right.
It may or may not be included in the halogen group.
Reason?
I’ll explain to you with some proof.
Look, first of all the Astatine element is a laboratory made radioactive element.
And it has a very short half life. The longest half life of a stable isotope Astatine-210 is 8.1 hours and of Astatine-211 is 7.2 hours.
That means half of the Astatine element will decay in just few hours, which produces heat. And that is why it is very difficult to conduct experiments on it.
The only way to conduct the experiment is to do so in either gaseous phase or in the dilute solution which absorbs the heat.
But the experiments conducted using such less quantity of elements may not give satisfactory results.
These experiments concluded that Astatine forms HAt, various salts like LiAt, interhalogens like AtI, and other halogen like bonds. (You can get this proof of experiments from google books here)
All these experiments clearly indicate that Astatine shows behavior similar to that of halogens.
But unfortunately, this is only partially true.
Because it has been found that astatine also forms At+ ions in an acidic solution.
Some researchers have found that pure astatine shows metallic characteristics in condensed phase. (Here is a research work by researchers: condensed astatine)
Also according to Cotton and Wilkinson, astatine shows more characteristics of halogens.
Hence on a newly published Periodic table, astatine is classed as a halogen.
(Note: Astatine also shows some metallic characteristics. If any authoritative sources are found further, then this article will be updated)
Properties of halogens
The physical and chemical properties of halogens (group 17 elements) are mentioned below.
Physical properties of halogens
#1 Halogens (group 17 elements) are nonmetals. And they are bad conductors of heat and electricity.
#2 Group 17 (halogens) on the Periodic table is the only group which contain elements in all the three phases i.e solid, liquid as well as gaseous phase
#3 Fluorine (F) exists in a gaseous state and it has a pale yellow color.
#4 Chlorine (Cl) exists in a gaseous state and it has a yellowish greenish color.
#5 Bromine (Br) is a dark red liquid and it forms a brown vapour on heating.
#6 Iodine (I) is a dark black color element in solid state and it forms purple vapour on heating.
#7 Astatine (At) is a laboratory made synthetic halogen which has a very short half life and it appears as a black solid.
#8 The melting point and boiling point of the halogens (group 17 elements) increases as we move down in the group.
#9 The atomic size of the halogens increases as we move down the group (from top to bottom).
#10 Density of halogens increases down the group (from top to bottom).
Chemical properties of halogens
#1 Halogens or group 17 elements are highly reactive nonmetals.
#2 Halogens have 7 electrons in their outermost orbit and they easily gain one electron to form a stable octet.
#3 All the halogen elements are poisonous.
#4 All the halogens molecules are diatomic molecules.
#5 The most reactive nonmetal on the entire Periodic table is Fluorine (F).
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Summary
In the beginning of this article we discussed “Where are the halogens located on the periodic table?”. We saw that halogens are found in the 17th group of Periodic table (exactly on the left of Noble gases).
Then we saw the exact reason why halogens (group 17 elements) are called so?
We know that halogens are the most reactive nonmetals. But amongst these halogens, fluorine is a highly reactive element on the entire Periodic table.
Then we discussed whether Astatine should be considered a halogen or not.
Finally we saw the physical and chemical properties of halogens (group 17 elements).
I hope you have found this article helpful for solving your doubts.
Let me know in the comments below, what you think about this article.
Suggested Important articles for you:
- Periodic table (with everything you need to know) (Important)
- Metals on the periodic table
- Nonmetals on periodic table
- Metalloids on periodic table
- Alkali metals on periodic table
- Alkaline earth metals on periodic table
- Noble gases on periodic table
- Transition metals on Periodic table
- Inner transition metals on periodic table
- What do elements in the same group have in common
