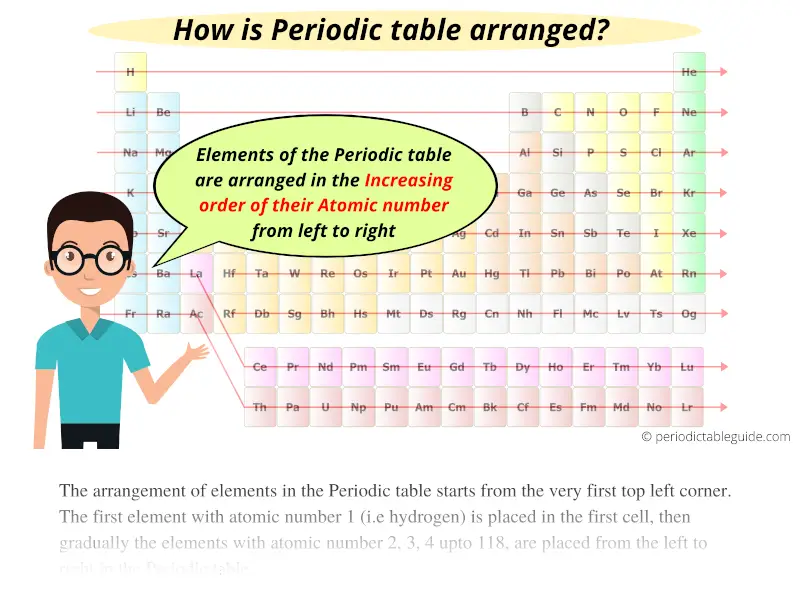
The Periodic table elements are arranged in the increasing order of their atomic number.
The arrangement of elements in the Periodic table starts from the very first top left corner.
The first element with atomic number 1 (i.e hydrogen) is placed in the first cell, then gradually the elements with atomic number 2, 3, 4 upto 118, are placed from the left to right in the Periodic table.
But hold on… this was just a short answer.
There are few important things which you must know regarding the arrangement of Periodic table.
Here I have covered important topics like;
- Why is Periodic table arranged by atomic number?
- What exactly is the atomic number? And what are protons?
- Step by step arrangement (from 1st to 118th element)
- Vertical columns (What are they?)
- Horizontal rows (What are they?)
- 4 blocks of Periodic table
Let’s finish these topics very quickly (you will surely get valuable information.)
Why is Periodic table arranged by Atomic number?
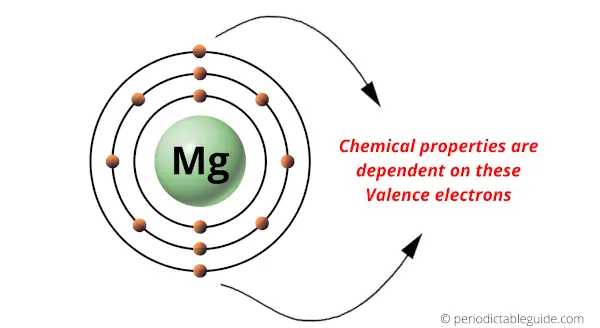
Periodic table is arranged by Atomic number because of electrons present in the outermost orbit (which are responsible for the chemical properties of the elements.)
Explanation:
To understand the reason behind this arrangement, let me tell you a story behind this.
During the year 1869, only 63 elements were known. [1]
Mendeleev arranged these known elements in the increasing order of their atomic masses.
And the Mendeleev’s Periodic table was something like this (as shown below.)
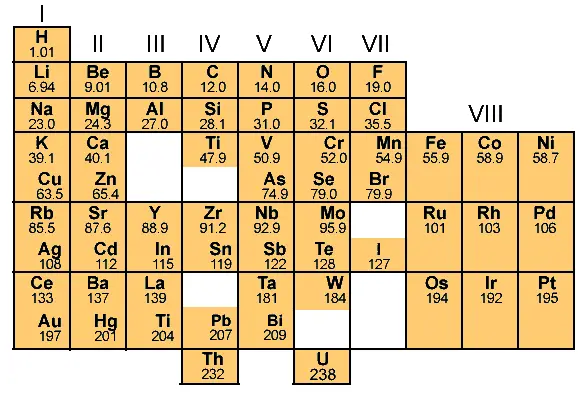
But during those years, there was no knowledge about the atomic structure.
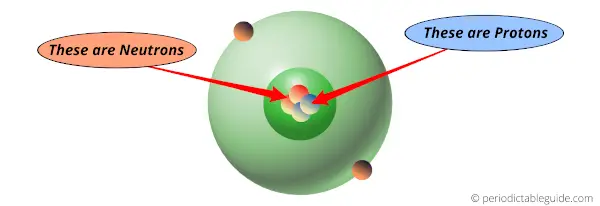
Later on in 1911, Rutherford came up with a discovery of atomic structure, and he found that there are protons and neutrons in the central part of atoms (In other words, Rutherford found that there is a nucleus in the central part of atom which consists of protons and neutrons.)
And the electrons revolve around the nucleus of the atom.
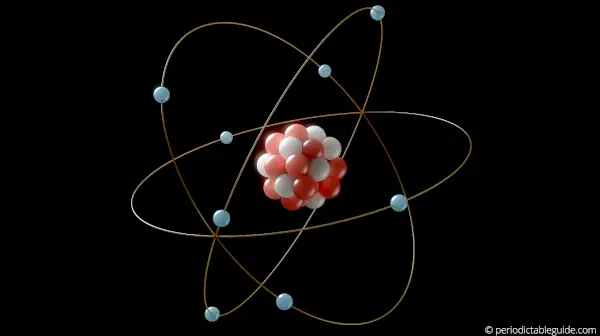
After the discovery of atomic structure, it was found that the chemical properties of elements are highly dependent on the number of electrons present in the outermost orbit.
Hold on …
You will come to know everything, but let us first understand few things about protons and atomic number.
What exactly are the protons and how are they related to the atomic number?
Protons are present inside the nucleus of an atom.
Protons are the unique identity for each element.
All the elements have a unique number of protons.
No elements have the same number of protons.
For example, the helium element has 2 protons, that means no other elements will have 2 protons except helium.
Carbon has 6 protons, that means no other elements will have 6 protons except carbon.
Thus, the number of protons is a unique number for each and every element.
Just as your ID proof is your unique identity, no other person can have the same ID proof as yours.
Similarly, each element has a unique number of protons in their nucleus. No other element can have the same number of protons in their nucleus.
Also in the modern periodic table, the number which you can see on the top is nothing but the number of protons (which is also known as atomic number.)
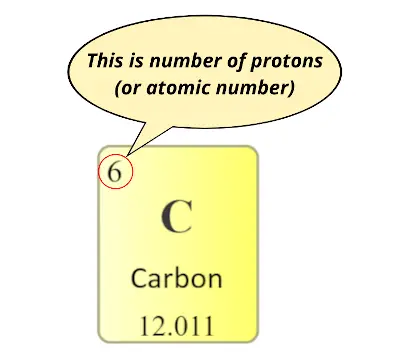
Now the question is;
What is a relationship between number of protons and number of electrons?
Simple answer: In a neutral atom, the number of protons and number of electrons are equal.
For example, this is a neutral helium atom.

Here you can see that the helium atom has 2 protons and the number of electrons are also 2.
Hence, for any neutral atom;
Number of protons (or atomic number) = Number of electrons
Summary (Why is Periodic table arranged by atomic number?)
So far in the above discussion, we have seen that the atomic number represents the number of electrons of an element.
Atomic number = Number of electrons
The electrons present in the outermost orbit represent the chemical properties of the elements.
Hence to classify the elements on the basis of similarities in their chemical properties, they are arranged in the Periodic table on the basis of atomic number.
In other words, Atomic number indicates the number of electrons, which are responsible for the chemical properties of elements.
Hence the Periodic table is organized on the basis of Atomic number.
Step by step arrangement (from 1st to 118th element)
So now you know that the elements are arranged according to the increasing order of their atomic number.
The first element having the least atomic number 1 (i.e hydrogen) is placed on the first cell of the Periodic table.
Further, the elements with Atomic numbers 2, 3, 4, 5, upto 118 are placed from left to right in the Periodic table as shown below.
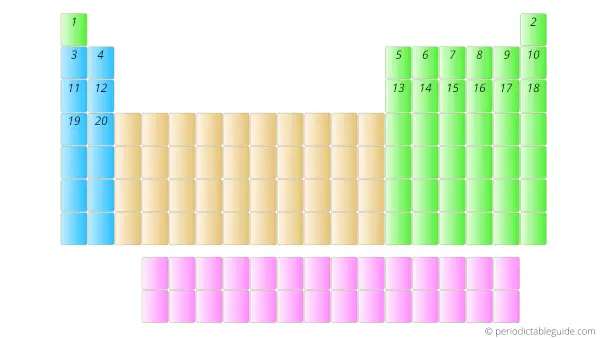
Now here one important thing is that, the elements having atomic number 58 to 71 and 90 to 103 are placed in separate rows at the bottom of the Periodic table.
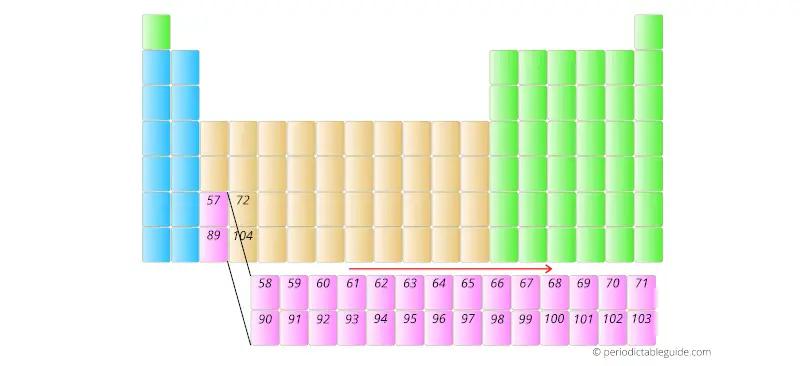
These elements are placed in separate rows at the bottom of the Periodic table because they differ in the chemical properties, plus by placing them at the bottom, the Periodic table shape does not distort.
They are known as inner transition metals.
Read more about: Inner transition metals
Vertical columns (What are they?)
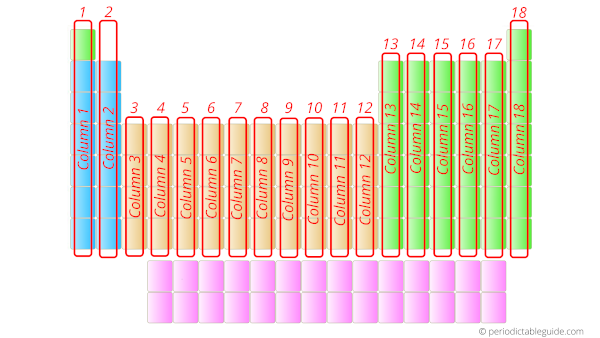
What are the vertical columns in the Periodic table?
Answer: They are groups of Periodic table.
There are total 18 vertical columns which are known as groups.
The elements which are in the same group have similar chemical properties.
Horizontal rows (What are they?)
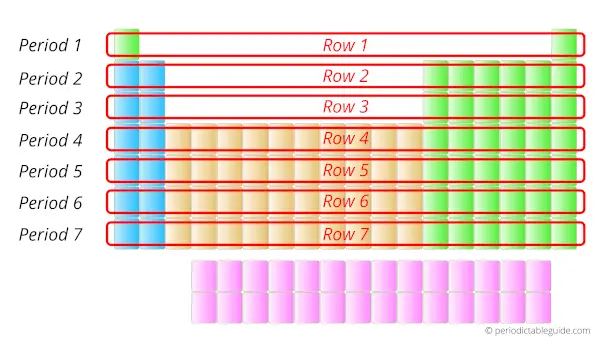
What are the vertical columns in the Periodic table?
Answer: They are the Periods of Periodic table.
Elements lying in the same period have the same number of orbits.
4 blocks of Periodic table
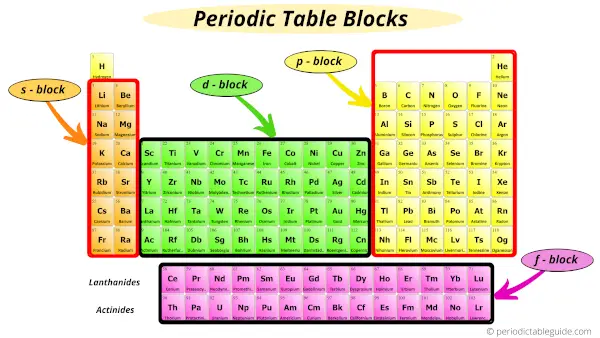
The elements of the Periodic table are also classified block wise.
There are 4 blocks in the Periodic table: s block, p block, d block and f block.
The elements which are in the s block have the valence electrons in s-orbitals.
Similarly, elements which are in the p block have the valence electrons in p-orbitals.
Elements in d block have valence electrons in d-orbitals and elements of f block have valence electrons in f-orbitals.
Final words
In the very beginning of this article, we have seen that the elements of periodic table are arranged in the increasing order of their atomic number.
And for a neutral atom, the number of protons are equal to the number of electrons.
These electrons are responsible for the chemical properties of an element.
Hence for grouping the elements according to the similar chemical properties, the elements of the Periodic table are arranged in the increasing order of their atomic number.
Later on, we saw that there are groups as well as periods on the Periodic table.
There are 4 blocks on the Periodic table which describes the position of valence electrons in the s, p, d or f orbitals.
I hope you have completely understood the arrangement of Periodic table.
Let me know in the comments, has this article helped you or not?
Free Gift for you: Interactive Periodic Table
Let me tell you how this Interactive Periodic Table will help you in your studies.
1). You can effortlessly find every single detail about the elements from this single Interactive Periodic table.
2). You will get the detailed information about the periodic table which will convert a newbie into pro.
3). You will also get the HD images of the Periodic table (for FREE).
Checkout Interactive Periodic table and download it’s high resolution image now (It’s FREE)