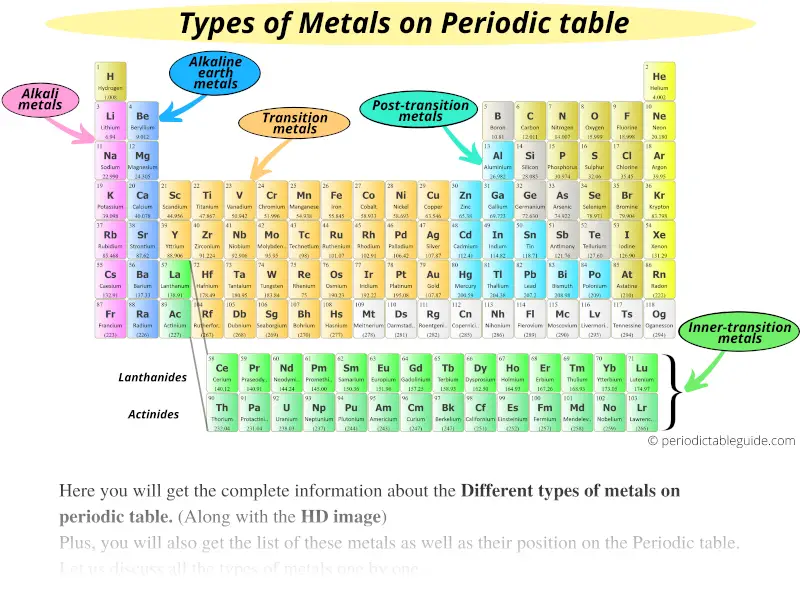
Here are the Different types of metals on the Periodic table.
They are;
- Alkali metals
- Alkaline earth metals
- Transition metals
- Inner transition metals
- Post transition metals
- Rare earth metals
- Heavy metals
I will give you a short introduction about these metals.
+
You will also get the list of these metals as well as their position on the Periodic table.
So let’s dive straight into it.
Types of metals on the Periodic table
Let’s see few important things for all the different types of metals on the Periodic table.
#1 Alkali metals
Alkali metals are located on the left most side of the Periodic table in group 1.
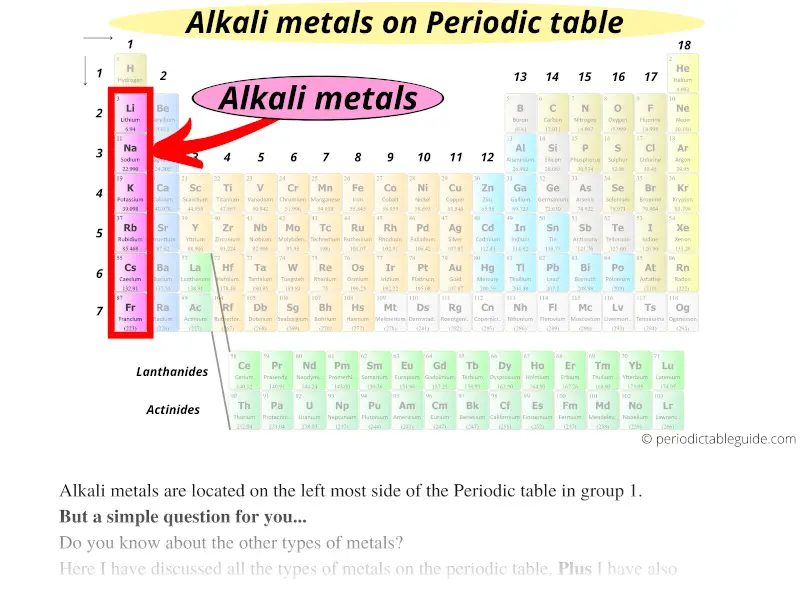
Alkali metals are the most reactive type of metals from the entire Periodic table of elements.
As we move down the group from top to bottom in the group 1, the reactivity of alkali metals increases.
List of alkali metals with atomic number, symbol and name.
Also read: What exactly are the Alkali metals and why are they called so?
#2 Alkaline earth metals
Alkaline earth metals are located on the left side of the Periodic table in group 2.
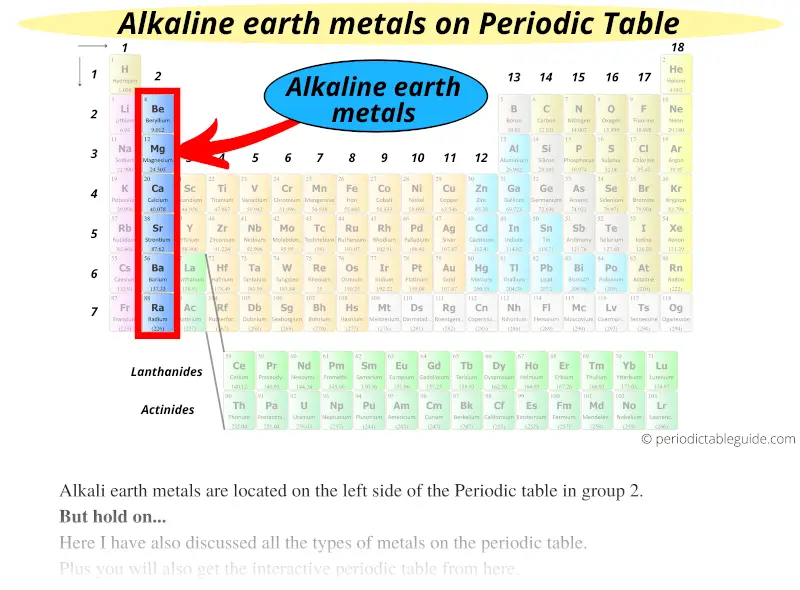
Alkaline earth metals are also the reactive type of metals but they are less reactive as compared to alkali metals.
All the alkaline earth metals have 2 electrons in their outermost orbit.
They are reactive metals and as a result, they are not found freely in nature, but they always exist in the form of compounds with other elements.
List of Alkaline earth metals with atomic number, symbol and name.
| Atomic number | Symbol | Element name |
| 4 | Be | Beryllium |
| 12 | Mg | Magnesium |
| 20 | Ca | Calcium |
| 38 | Sr | Strontium |
| 56 | Ba | Barium |
| 88 | Ra | Radium |
Also read: What exactly are the Alkaline earth metals and why are they called so?
#3 Transition metals
Transition metals are found in the middle part of the Periodic table (from Group 3 to group 11).
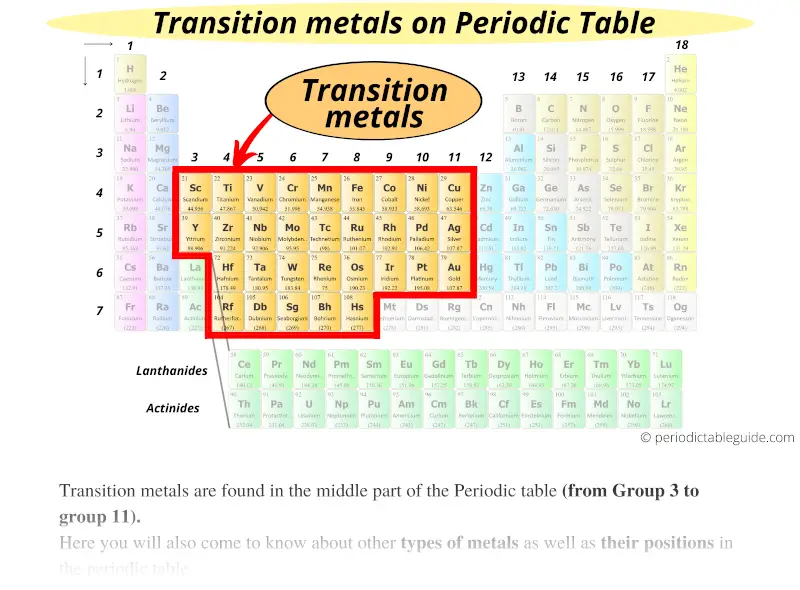
But the question is,
Do you know why transition metals are called transition metals?
Here is a short answer: Transition metals (also known as Transition metals) are those types of metals whose properties show the transition from electropositive s-block elements to the electronegative p-block elements.
They form a bridge between the chemically active metals of s-block elements and the less active elements of Groups 13 and 14.
Thus these elements are known as “Transition metals”.
Also see:
1). Electron configuration of transition metals (List).
2). How many transition metals are there? And why group 12 elements are excluded from transition metals?
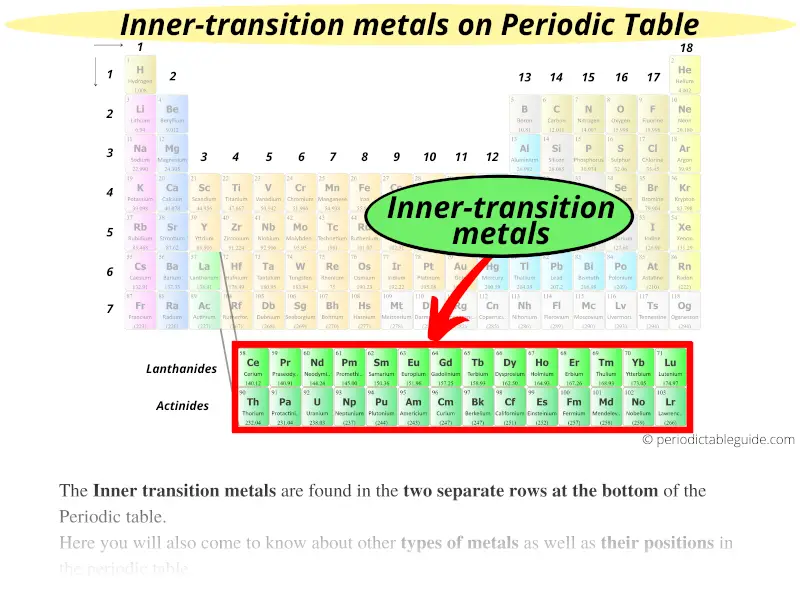
#4 Inner transition metals
The inner transition metals are found in the two separate rows at the bottom of the Periodic table.
Now here is a question for you,
Why are they called inner transition metals?
The answer is: These elements have somewhat similar properties like that of transition metals, plus they are the elements of group 3 only, but they are placed at the bottom of the Periodic table as the inner section of group 3.
Hence they are known as inner transition metals.
Also read: The simple reason why inner transition metals are placed at the bottom of Periodic table.
#5 Post transition metals
Universally accepted Post transition metals as shown in the below image and are located between the transition metals and the metalloids.
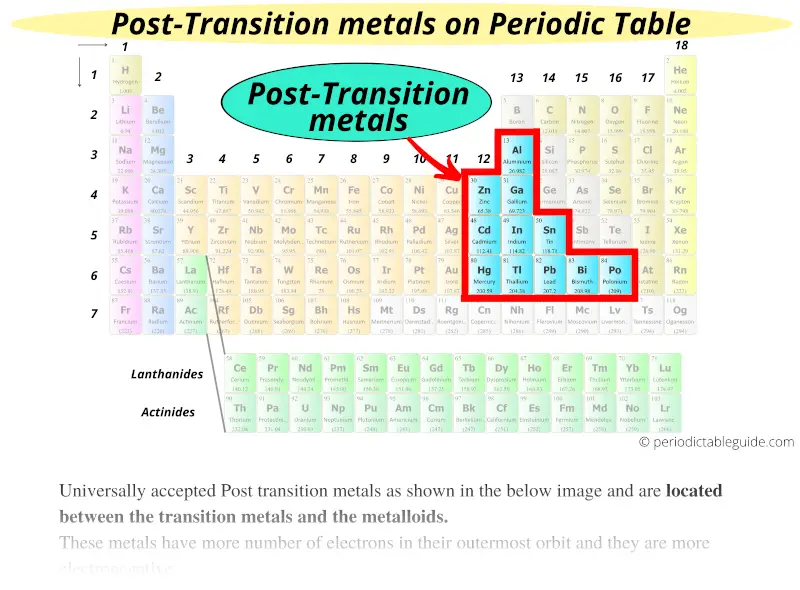
These metals have more number of electrons in their outermost orbit and they are more electronegative.
They are rich in electrons which indicates that they generally form soft cations.
They have lower melting point and are brittle as compared to other metals.
Based on these above mentioned properties, they are also known as other metals or poor metals.
#6 Rare earth metals
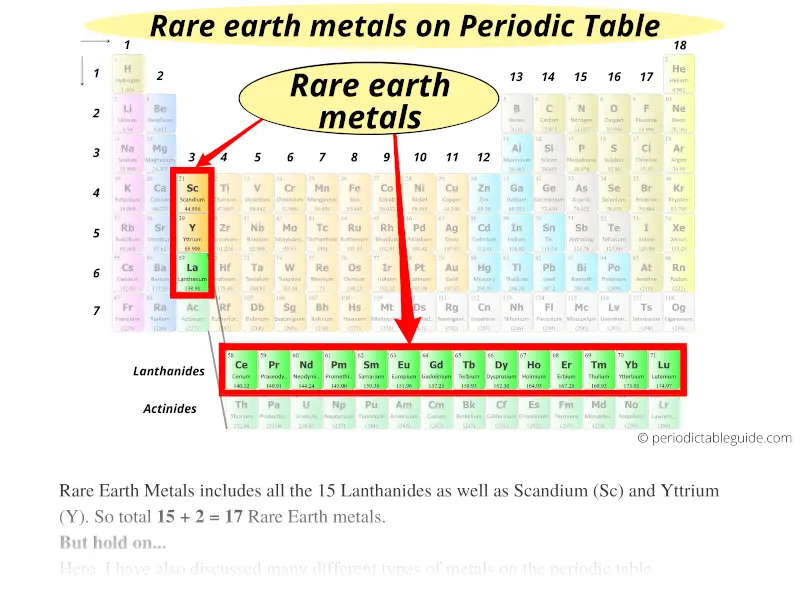
There are total 17 Rare Earth metals on the Periodic table. Rare Earth Metals includes all the 15 Lanthanides as well as scandium (Sc) and yttrium (Y). So total 15 + 2 = 17 Rare Earth metals.
If you are thinking that rare earth metals are those types of metals on Periodic table which are very rarely available, then you are WRONG.
Read here: Reason why Rare Earth metals are called so.
#7 Heavy metals
Heavy metals are those metals which have higher density or higher atomic mass.
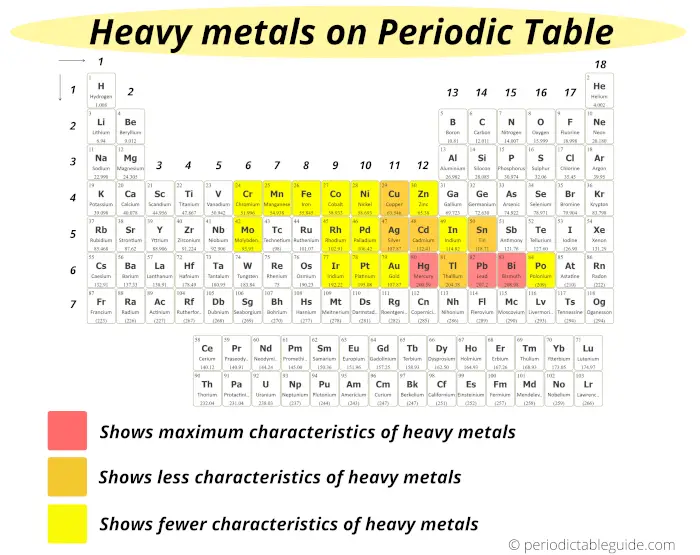
The metals which show maximum characteristics of heavy metals are;
These heavy metals are displayed on the Periodic table with red color (see above image)
The metals which show less characteristics of heavy metals are;
And these elements are represented by orange color on the Periodic table.
Rest of the elements which are shown in yellow color show fewer characteristics of heavy metals.
Also see:
1). The complete list of all the metals on the Periodic table.
2). Why are metals on the left of Periodic table?
I hope you have understood the different types of metals on the Periodic table.
If you have any queries, feel free to ask me in the comments below.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Suggested Important articles for you:
- Periodic table of elements (Detailed guide + HD image)
- Periodic table with nonmetals
- Periodic table with metalloids
- Periodic table with halogens
- Periodic table with noble gases
- What does the group number and period number tell you about the element?
- List of 118 elements
- Periodic trends (Trends in periodic table)
