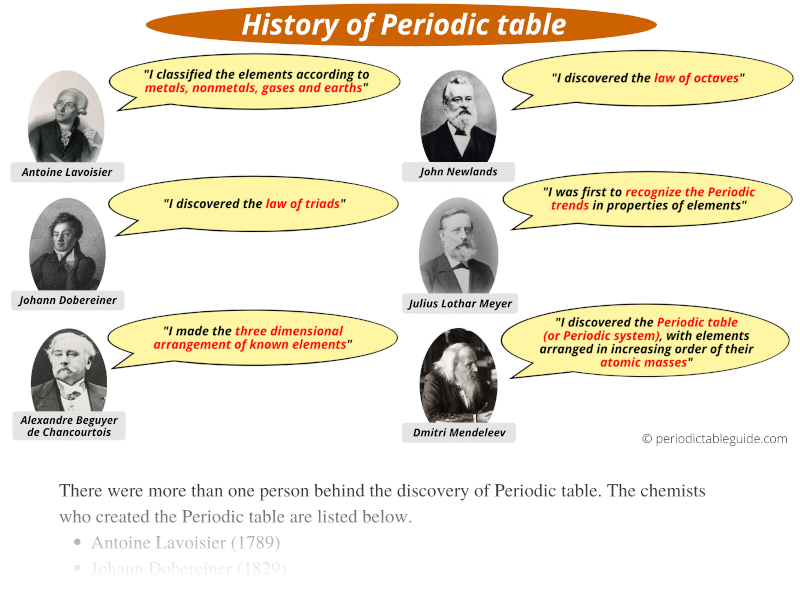
History of Periodic table:
It was not the one person (Dimitri Mendeleev) behind the discovery of Periodic table.
But it was the combined efforts of many chemists for the invention of Periodic table.
The chemists who invented Periodic table are listed below.
- Antoine Lavoisier (1789)
- Johann Dobereiner (1829)
- Alexandre Beguyer de Chancourtois (1862)
- John Newlands (1864)
- Julius Lothar Meyer (1870)
- Dmitri Mendeleev (1869)
- Henry Moseley (1913)
- And many more…
Hold on…
I’ll tell you the complete History of Periodic table starting from 1789 to 1913.
But before that, it is very very important to know the reason why periodic table was invented.
Why Periodic table was invented?
The Periodic table was invented in order to classify all the known elements according to the similarities in their properties.
Explanation:
Let me explain this with an example which you already know.
There are so many elements that exist in nature.
Till today there are total 118 known elements like hydrogen, helium, lithium, beryllium, boron, carbon, and so on…
All these elements have different properties.
Some are solids, some are liquid, some are gases, some are metals, some are nonmetals, some are highly reactive while some are less.
In short their physical and chemical properties are different from each other.
There are just a few elements in the above image, but if you want to study all the 118 elements, then just imagine how difficult it would be for you to study them. Very difficult. Isn’t it?
So in order to study all these elements, they are divided into small groups.
Let me give you an example.
If you have visited any shop, then you might have noticed that all the similar products are kept together.

Above image clearly indicates that all the similar products are kept in their individual groups.
Why are such arrangements done in shops?
Just imagine that you are going in a shop to buy Coca-Cola.
Now what if all the products are messed up here and there in the shop?
It will be difficult for the shopkeeper to find a Coca-Cola for you. Isn’t it?
So for the ease of finding Coca-Cola and other products, they are properly arranged in the shop.
Same thing needs to be done with the 118 elements also.
All the elements have different physical and chemical properties.
But there are many elements which show similar properties.
By grouping these elements in each individual group, it becomes very easy to study their properties, uses and many more.
Hence for the ease of understanding the elements and their properties, they are classified in different groups and periods on the basis of their similarities.
I hope you have now understood the reason why Periodic table was invented.
Who invented Periodic table? (The complete history of Periodic table)
It was not the efforts of a single person (i.e Dimitri Mendeleev) behind the invention of Periodic table. It was the combined efforts of many scientists who contributed to the discovery of the Periodic table.
Following chemists gave their valuable efforts in creating the Periodic table.
- Antoine Lavoisier (1789)
- Johann Dobereiner (1829)
- Alexandre Beguyer de Chancourtois (1862)
- John Newlands (1864)
- Julius Lothar Meyer (1870)
- Dmitri Mendeleev (1869)
- Henry Moseley (1913)
- And many more…
Many of you might think that Mendeleev was the person who discovered the Periodic table.
But this is not completely true.
Yes, he was the first to publish the Periodic table with 63 elements which were discovered during his time. (He published a Periodic table which was arranged on the basis of atomic masses of elements.)
But the question is;
Does Mendeleev deserve all the credit for his discovery?
Probably NO.
Here is the detailed reason behind it.
Let’s see their contribution of all the chemists one by one from the year 1789 to 1913.
Antoine Lavoisier

In 1789, Antoine Lavoisier classified the known elements in the categories of;
- Metals
- Nonmetals
- Gases and
- Earths
He classified the known elements by knowing their properties.
After few years of this classification, several attempts were also made by other chemists.
But some important work was given by Johann Dobereiner after few years.
Let us see how Johann Dobereiner contributed to the development of Periodic table.
Johann Dobereiner

In 1829, Johann Dobereiner discovered the law of triads.
During his time 30+ elements were known and he arranged these known elements in the increasing order of their atomic masses.
He prepared the triads (group of three elements) of a few elements as shown below.
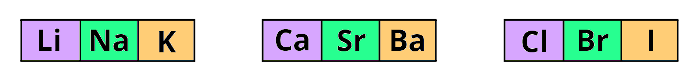
He found that the atomic mass of the middle element is the average of the atomic masses of 1st and 3rd elements.
But this was not applicable for all the elements.
Alexandre Beguyer de Chancourtois

Alexandre Beguyer de Chancourtois was a French geologist.
In 1862, he gave his contribution in the invention of Periodic table by discovering the three dimensional arrangement of chemical elements.
He published the new arrangement of known elements on a cylindrical shell in a helical path.
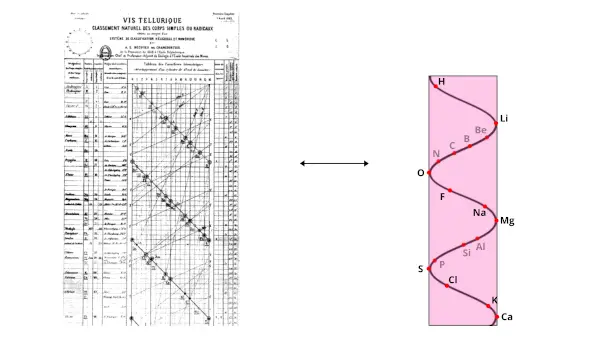
He arranged the elements in a helical path on the surface of cylinder in the order of increasing atomic mass.
He arranged these elements in such a way that one complete turn indicates the increase of atomic weight by 16.
According to Alexandre Beguyer de Chancourtois, the elements lying in the same vertical line on the cylinder shows the similar properties.
For example, in the above cylinder, you can see that Li, Mg and Ca are in the same vertical line.
So they show similar properties.
Again this arrangement of elements was not suitable for all the elements.
Now let us see John Newlands’s contribution in development of Periodic table.
John Newlands
In 1864, Newlands discovered the law of octaves.
There were only 56 elements known at his time.
He arranged these known elements in the increasing order of their ATOMIC MASS.
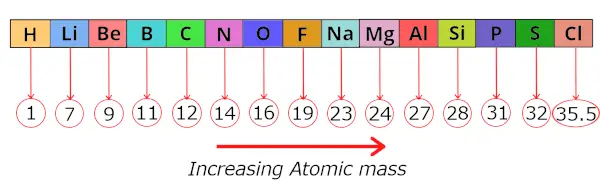
He found that after this arrangement, every 8th element shows a similar property as that of its respective 1st elements.
For example,
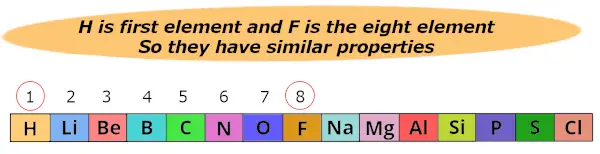
Here hydrogen (H) is 1st element and Fluorine (F) is the 8th element.
So Newlands found that these two elements show similar properties.
Thus he found that by arranging the elements in the increasing order of their atomic masses, every 8th element shows a similar properties.
But unfortunately his paper was not accepted because he had placed some dissimilar elements in one slot to maintain the pattern of similar properties.
Julius Lothar Meyer

Lothar Meyer studied the combining capacity of the known elements.
For example, if a copper atom combines with one oxygen atom, then its combining capacity is 1. If a copper atom combines with two oxygen atoms, then its combining capacity is two.
This way he found the combining capacity of the known elements. (Combining capacity of elements is also known as valency.)
He arranged the main group elements on the basis of their valency (or combining capacity).
Later in 1868, he included transition metals also in his table.
The elements in his table were arranged in the increasing order of their atomic masses, but he kept the elements with same valency in the vertical lines.
Lothar Meyer completed his table in 1870. But unfortunately his work was not accepted, because Mendeleev had already published a similar table of elements before 1 year in 1869.
Lothar Meyer was completely unaware about Mendeleev’s work.
He did not know that Mendeleev was also working on the same type of arrangement of elements.
Mendeleev had already published a table in 1869 which Lothar Meyer invented in 1870.
So finally Lothar Mayer accepted that he was late to publish his work.
But he contributed to the development of Periodic table in another way.
He was the first scientist to discover the Periodic trends in the properties of elements.
For known elements, he plotted the graph of atomic volume vs atomic mass, which shows a repeating pattern in their properties.
This way Lothar Meyer’s contribution is also praiseworthy.
Dmitri Mendeleev

In 1869, Mendeleev arranged the known elements in the increasing order of their atomic masses.
He prepared the cards of each known element with their properties and details written on them.
He arranged these cards from left to right in the increasing order of atomic mass.
If the property of the elements seems to be similar, he used to place it in the same column.
By doing so, he prepared the entire table and it looks something like this.
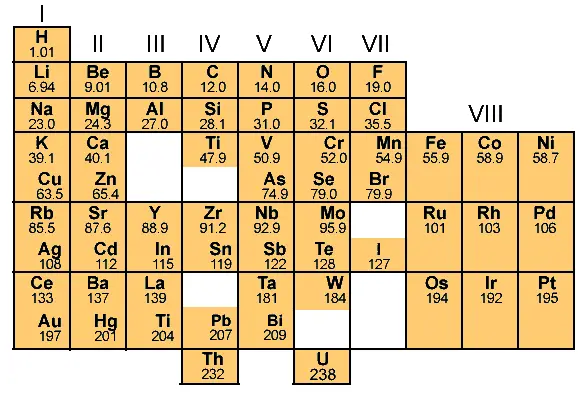
Do you know why Mendeleev left some gaps in his Periodic table? (See gaps in above image.)
The simple answer: While arranging the elements according to atomic masses, Mendeleev found that the properties of few elements were not matching with any of the previous ones, so he didn’t place such elements in that particular column.
So there were some empty gaps in his table.
Later on, these gaps were filled when the new elements were discovered.
For the great contribution in creation of Periodic table, Dmitri Mendeleev is also known as a father of Periodic table.
Henry Moseley

In 1913, Henry Moseley performed some experiments in which he bombarded X-rays on few samples of elements.
He noted down the emitted wavelength, and finally he (along with three other scholars) found that there was a direct relationship between wavelength and atomic number.
He plotted the graph of atomic number vs square root of X-ray frequency. And he found that the graph was a straight line.
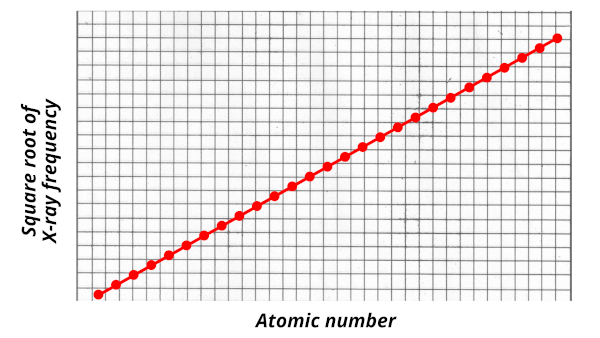
But this was not the end. Many other researchers contributed to the invention of Periodic table, but the final arrangement of elements was perfectly done on the basis of atomic number.
Here is how it was done.
Final arrangement (Based on atomic number)
After the valuable contribution of all the above mentioned scholars, many researchers worked on the atomic structure and finally the structure of atoms came into existence.
Scientists realized that there is a heavy nucleus in the centre of atoms. This nucleus contains protons and neutrons in it.
They also realized that the protons are the unique identity of every single element.
The number of protons present in the nucleus of an atom is known as atomic number.
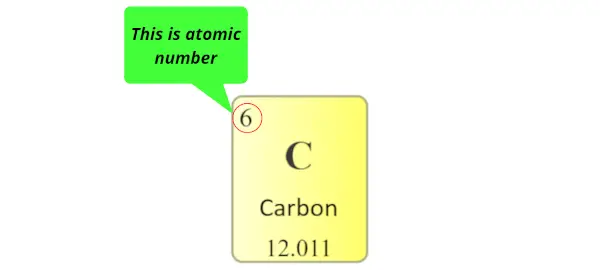
Each element has a unique number of protons in their nucleus. No other element can have the same number of protons in their nucleus.
Just like our ID proof is our unique identity. No other person can have the same ID proof as ours.
Based on this valuable research by different scholars, the elements in the Periodic table were finally arranged in the increasing order of their atomic number.
This way, we got our well arranged Periodic table of elements after so many years of contribution from many chemists.
I hope you have clearly understood the entire history of Periodic table along with the scientists who invented Periodic table of elements.
Let me know in the comments, whether this article helped you or not?
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Image references:
Antoine Lavoisier: CC BY 2.0, via Wikimedia Commons.
Johann Dobereiner: Carl August Schwerdgeburth, 1785-1878 (engraver), and Fritz Ries, 1826-1857 (painter), Public domain, via Wikimedia Commons.
Alexander Beguyer: Alexandre-Emile Béguyer de Chancourtois, Public domain via wikimedia commons.
John Newlands: DALIBRI, Public domain, via Wikimedia Commons.
Lothar Meyer: Wilhelm Hornung, Public domain, via Wikimedia Commons.
Dmitri Mendeleev: Via wikimedia commons.
Henry Moseley: AIP Emilio Segre Visual Archives, W. F. Meggers Gallery of Nobel Laureates, Public domain, via Wikimedia Commons.
