The group number tells you the total number of electrons present in the outermost orbit of an atom.
In other words, group number tells you the number of valence electrons of an atom.
For example,
Let us consider group 1 of the Periodic table.
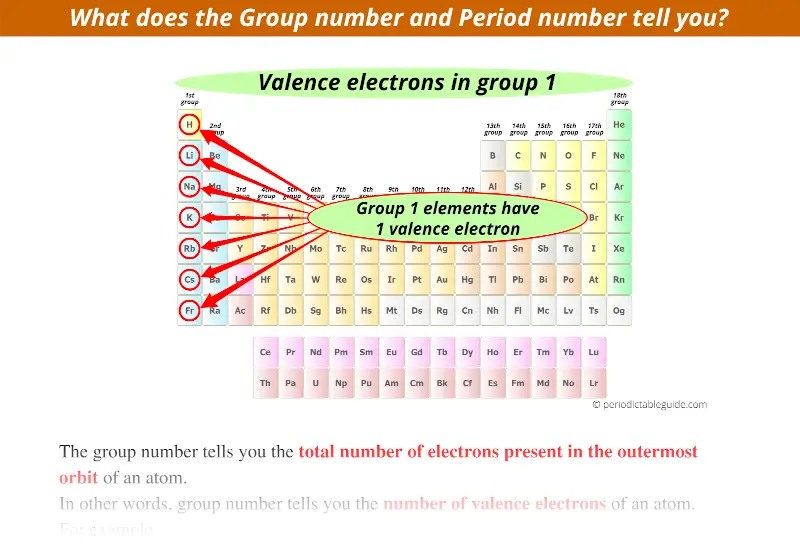
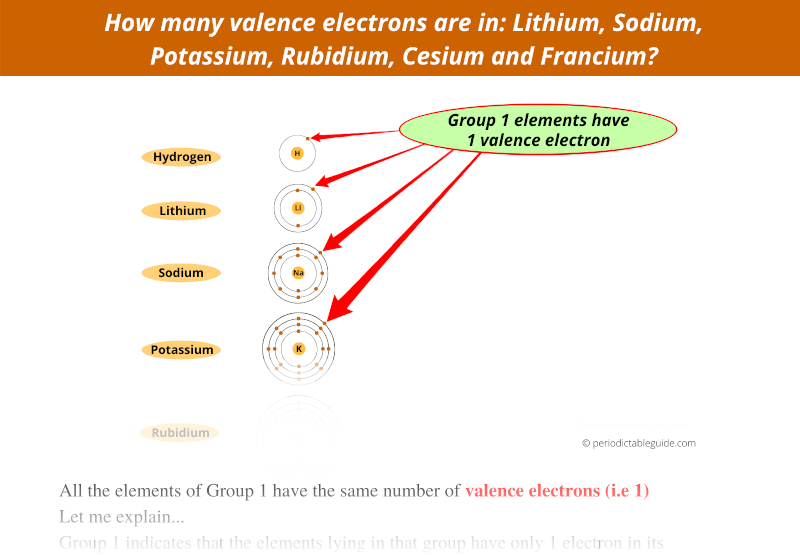
Group 1 indicates that the elements lying in that group have only 1 electron in its outermost orbit.
In other words, group 1 elements have 1 valence electron.
Similarly, consider group 2 of the periodic table.
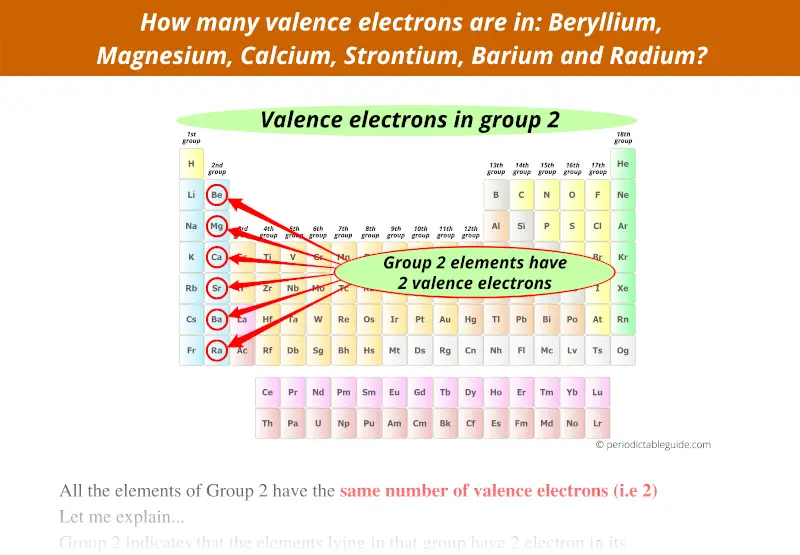
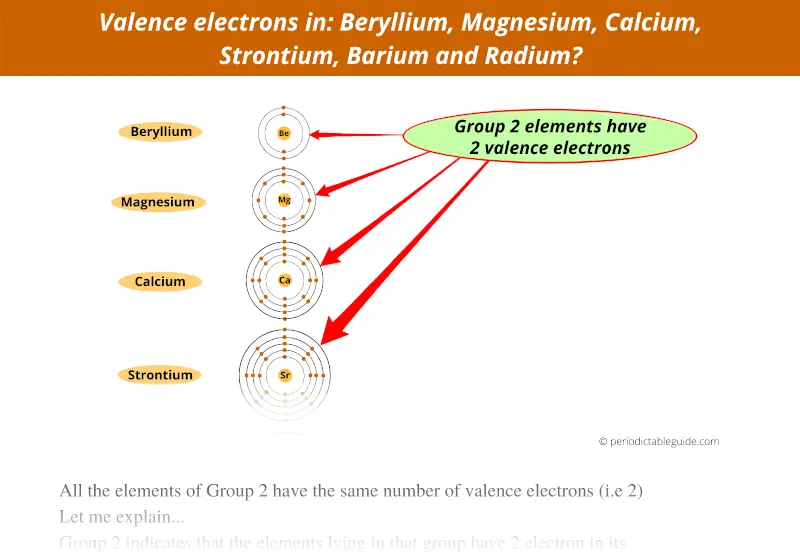
Group 2 represents that the elements lying in that group have 2 electrons in the outermost orbit.
Or we can say that these elements of group 2 have 2 valence electrons.
Similarly, also remember that;
Group 13 elements have 3 valence electrons, not 13.
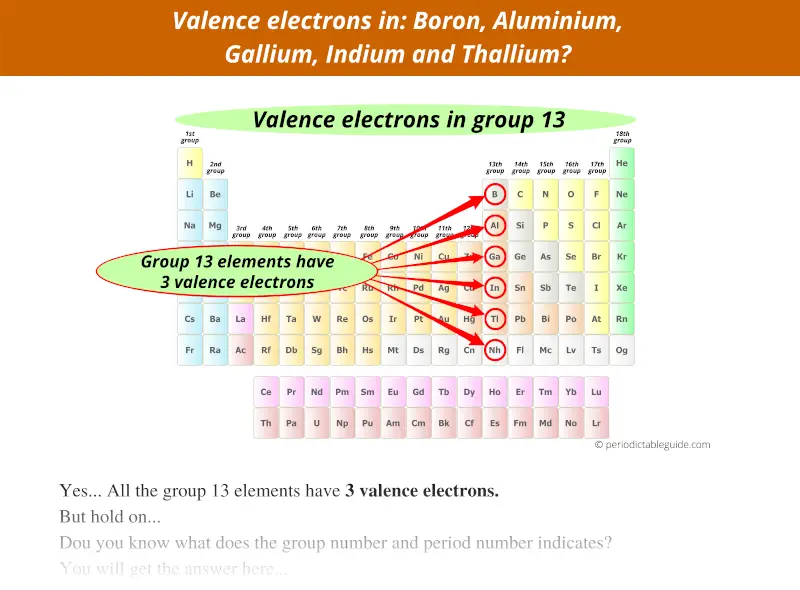
Group 14 elements have 4 valence electrons, not 14.
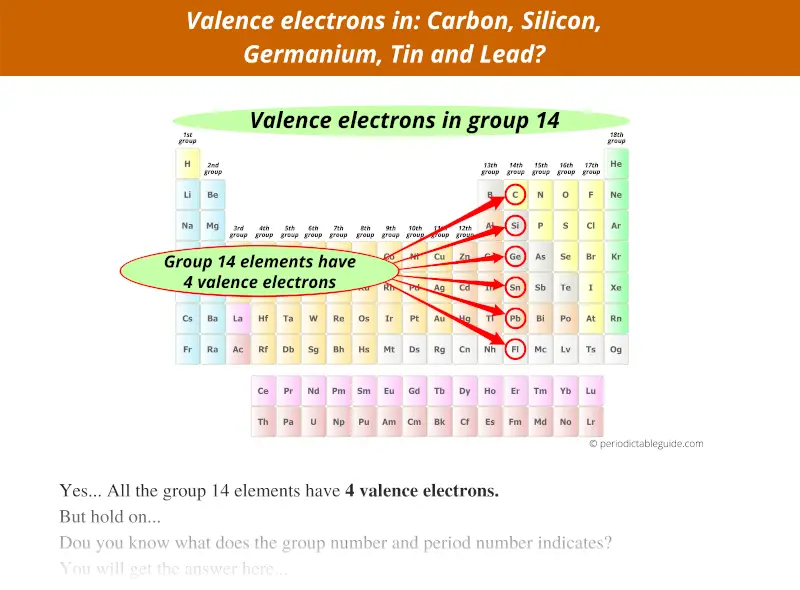
Group 15 elements have 5 valence electrons, not 15.
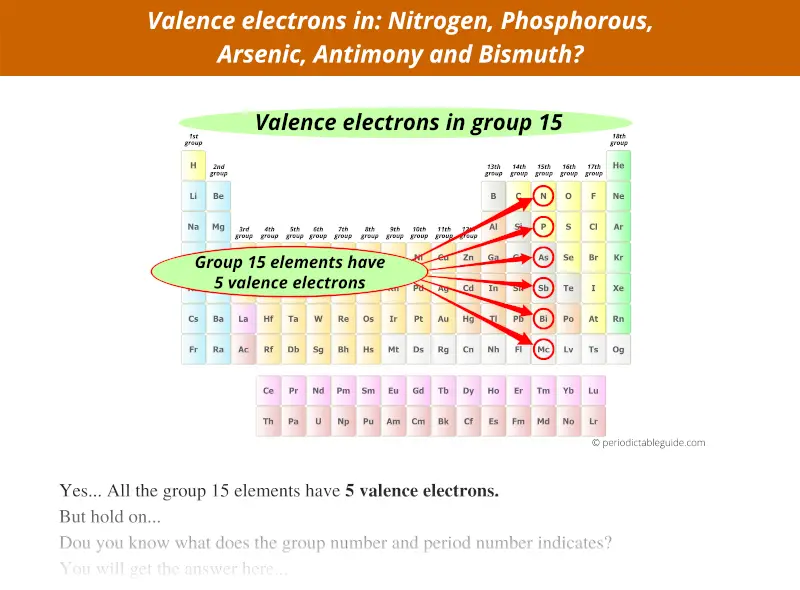
Group 16 elements have 6 valence electrons, not 16.
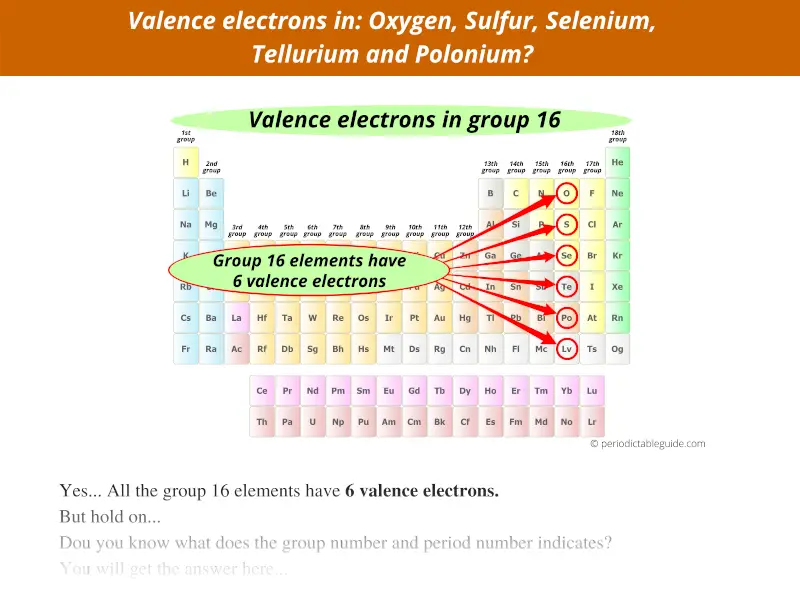
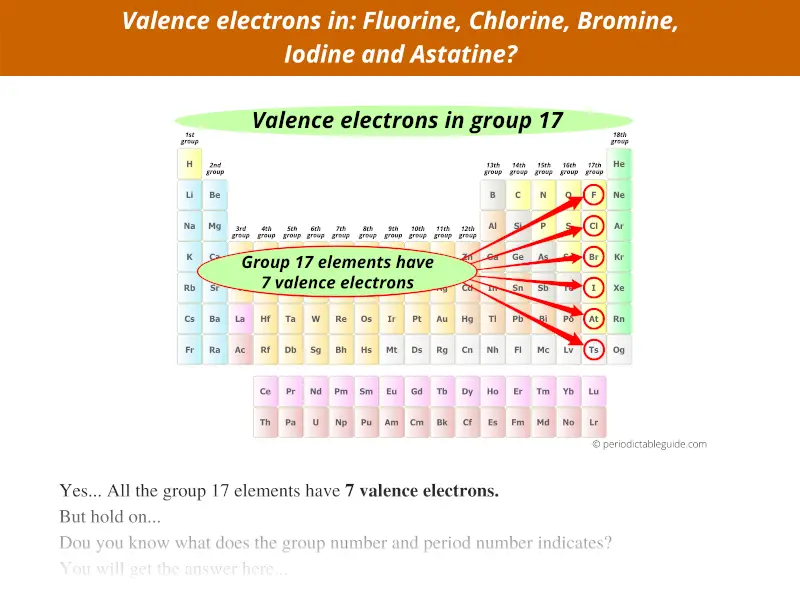
Group 17 elements have 7 valence electrons, not 17.
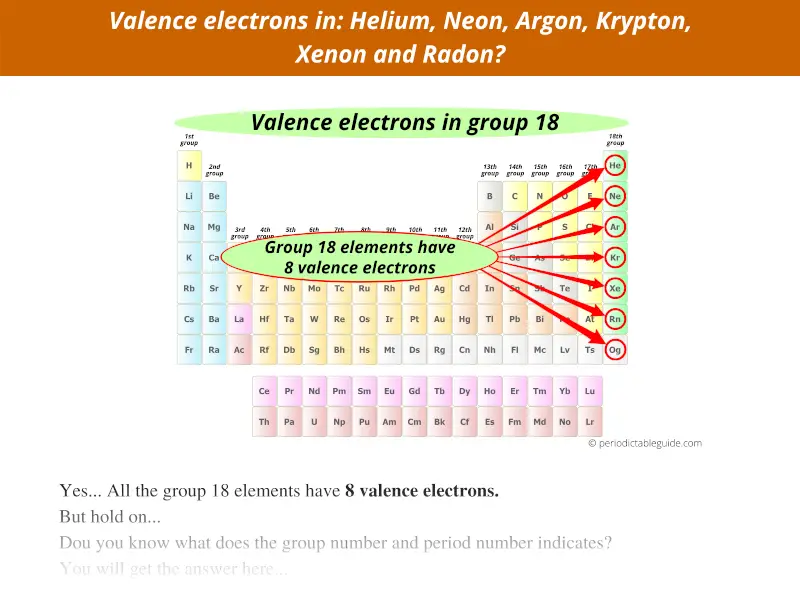
Group 18 elements have 8 valence electrons, not 18.
(Side Note: d-block elements and f-block elements do not show the same number of valence electrons as that of their group number.
For example, group 3 elements of d-block should have 3 electrons in outermost orbit. But they have 2 electrons in outermost orbit.
Similarly, group 4 elements of d-block should have 4 electrons in outermost orbit, but they also have 2 electrons in outermost orbit.
This is because the d-block elements have outermost electrons in s-orbitals as well as d-orbitals.
Plus these d-block elements have incomplete d-orbitals.
Thus electrons of both s-orbitals as well as d-orbitals participate in chemical reaction.
Similarly, f-block elements have incomplete f-orbitals.
And here also, the electrons of both s-orbitals and f-orbitals participate in chemical reaction.
Hence d-block and f-block elements have variable valency or different valence electrons other than the group number.)
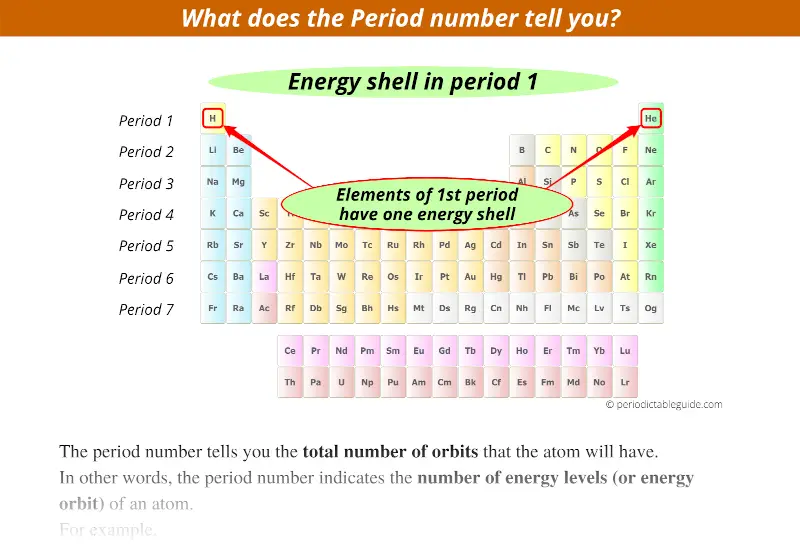
What does the period number tell you?
The period number on the Periodic table tells you the total number of orbits that the atom will have.
In other words, the period number indicates the number of energy levels (or energy orbit) of an atom.
For example,
1st period indicates that these elements possess 1 energy shell (or energy orbit).
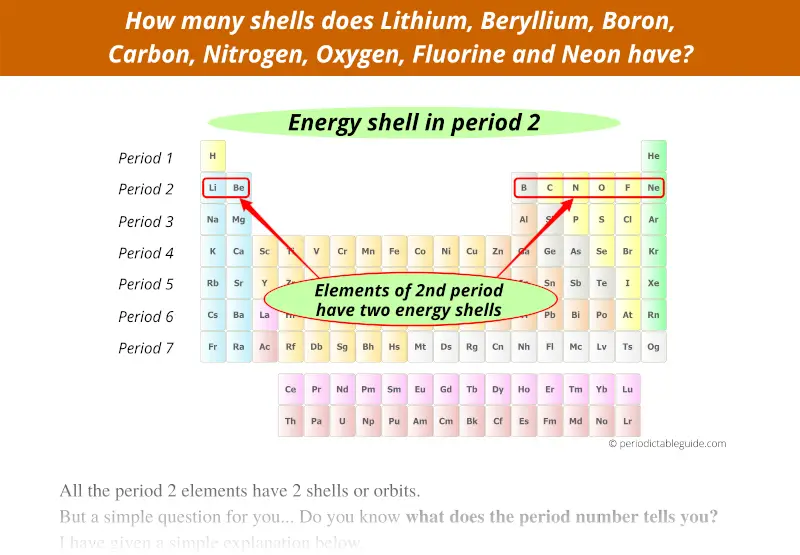
2nd period indicates that these elements possess 2 energy shells.
3rd period indicates that these elements possess 3 energy shells.
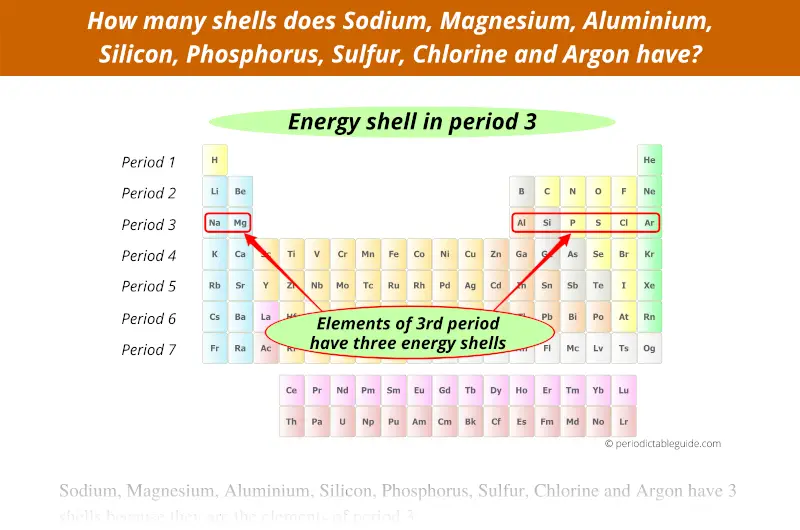
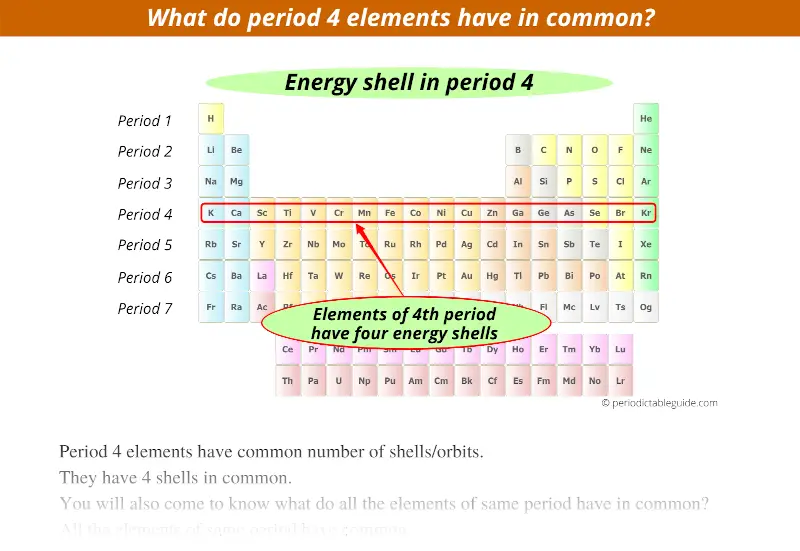
4th period indicates that these elements possess 4 energy shells.
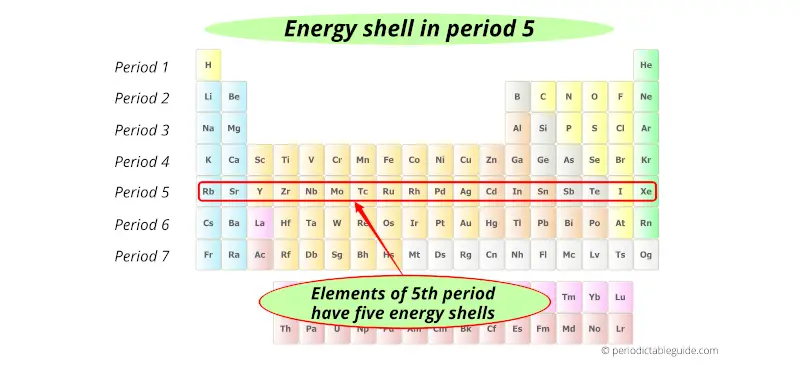
5th period indicates that these elements possess 5 energy shells.
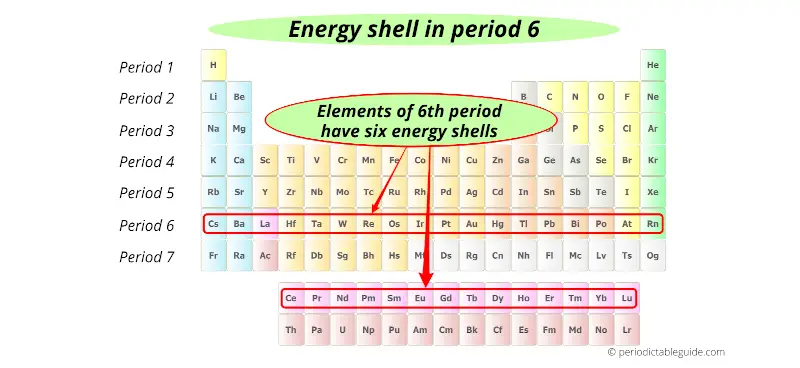
6th period indicates that these elements possess 6 energy shells.
And lastly,
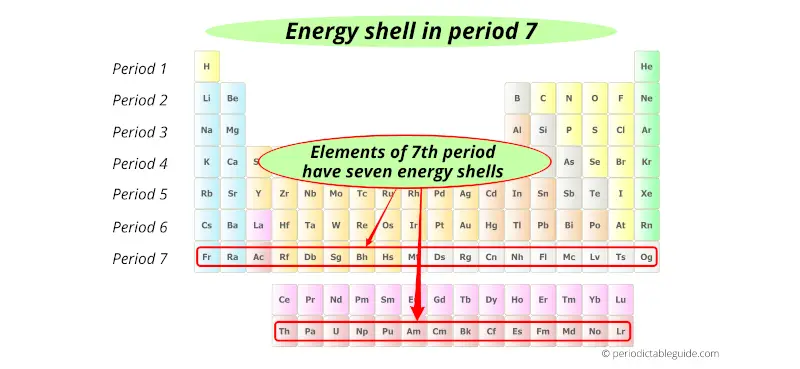
7th period indicates that these elements possess 7 energy shells.
Final words
The groups and periods are very important on the Periodic table.
The elements are arranged according to their atomic number on the modern periodic table.
The elements which are in the same groups have the same number of electrons in outermost orbit (i.e 1st group has 1 electron in outermost orbit, 2nd has 2 electrons in outermost orbit, 13th group has 3, 14th has 4, and so on…)
Also, the periods of periodic table indicates the number of energy orbits or energy shells of an atom.
Elements of period 1 have 1 energy shell, elements of period 2 have 2 energy shells, and so on…
This is it for this topic.
I hope now you know what group number and period number tells you about the atom on a Periodic table.
If you have any doubts, feel free to ask me in the comments below.
Also let me know, has this article helped you or not?
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Suggested Important topics for you:
- Periodic table of elements (Detailed guide + HD image)
- How are the elements arranged in the modern periodic table?
- Types of metals on Periodic table
- List of elements of Periodic table
- Metals on the periodic table
- Modern periodic table with atomic mass and atomic number
- Detailed Periodic table with electron configuration
- Periodic table with electron shells
- All Periodic trends in periodic table
