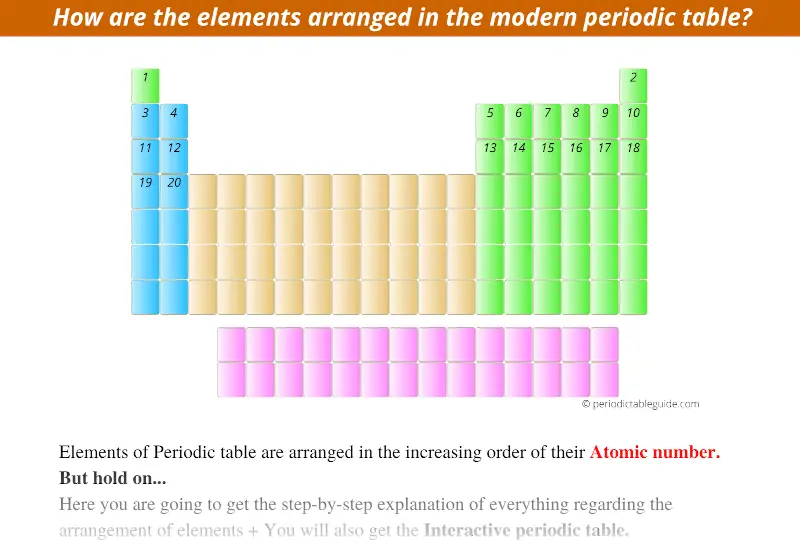
The elements in the Modern Periodic table are arranged in the increasing order of their Atomic Number.
Let me show you how they are arranged…
As shown in the above image, the element with a minimum atomic number is placed in the top-left corner of the Periodic table. This element is hydrogen having atomic number 1.
Starting from this first element, the other elements with atomic number 2, 3, 4, 5, and so on… are placed from left to right direction in the Periodic table. (See below image)
In this way, all the 118 known elements are arranged in the Modern Periodic table based on their atomic number.
But wait… one important thing.
The actual Periodic table looked something like this.
There are 32 columns and 7 rows in this Periodic table and is also known as long form Periodic table or 32 column Periodic table.
But in many books and school text books, there are 18 column Periodic table (as shown below).
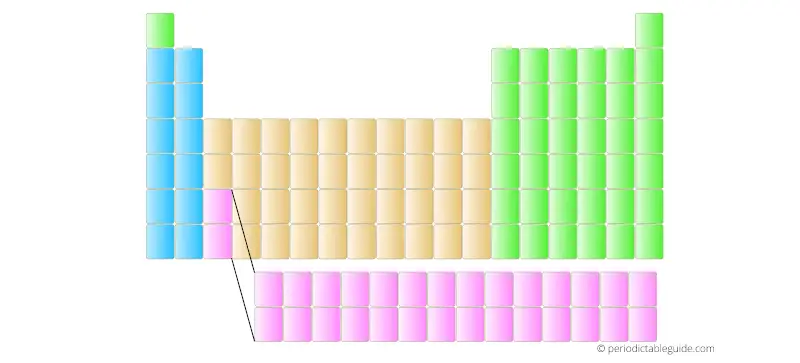
The two rows are placed at the bottom of the Periodic table in order not to distort the shape of Periodic table and also it makes the Periodic table fit perfectly on the A4 size paper.
Also the elements placed in the two bottom rows have different electron configuration and different properties. They are known as Lanthanides and Actinides. (Read more about the Lanthanides and Actinides from here)
Still confused?
Let me explain this very quickly.
The element with atomic number 57 is placed in the main part of the Periodic table as shown in below image.
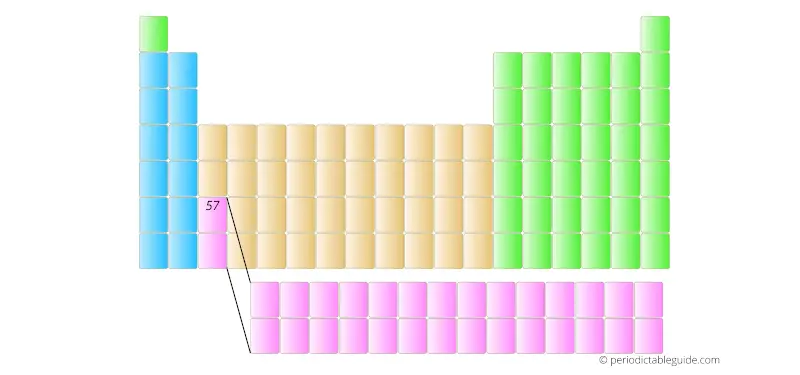
Further the elements having atomic number 58, 59, 60, etc are placed in separate rows at the bottom of the Periodic table.
This repeats upto element with atomic number 71.
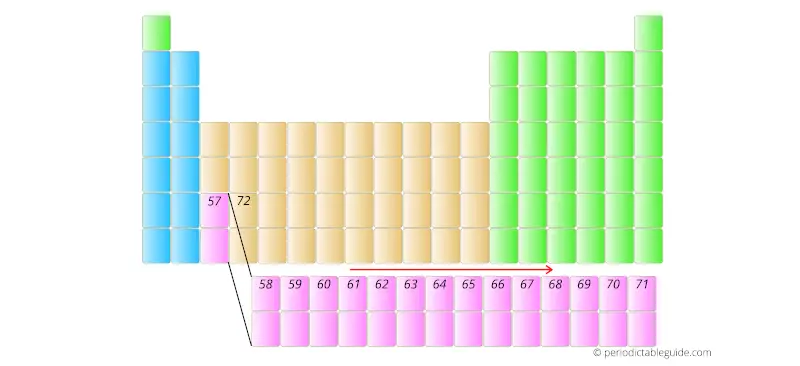
Similarly, the elements having atomic number 90 to 103 are placed in the other row as shown below.
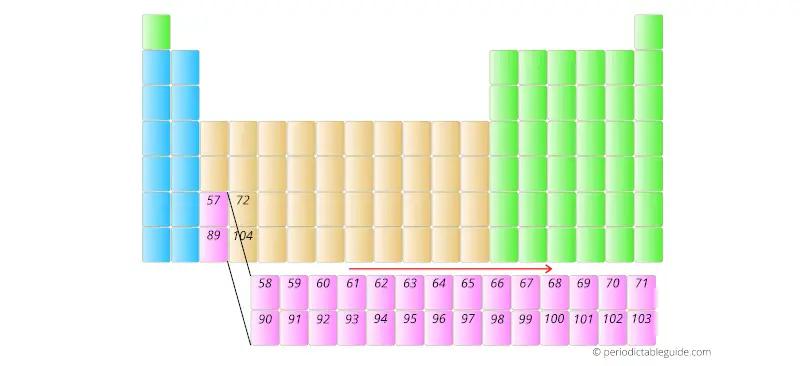
And the reason why they are kept in the bottom two rows is that they have different electron configuration and different chemical properties.
I hope now you have got few idea about the two rows lying at the bottom of the Periodic table.
Now let me give you some glimpse of rows and columns of the Periodic table.
Rows in Periodic table
There are total 7 rows in Periodic table.
The rows of the Periodic table are known as Periods.
The elements in the same row (period) have the same number of orbits.
For example,
Let’s take elements of period 3.
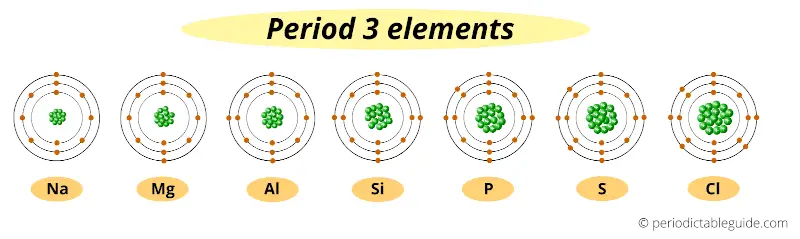
These are period 3 elements. You can see that all these elements have the same number of orbits (i.e 3 orbits).
Similarly,
Elements of 1st period have 1 orbit.
Elements of 2nd period have 2 orbits.
Elements of 4th period have 4 orbits.
Elements of 5th period have 5 orbits.
Elements of 6th period have 6 orbits and
Elements of 7th period have 7 orbits.
Columns in Periodic table
The columns of the Periodic table are called groups.
There are total 18 groups in modern Periodic table.
The elements of the same column (group) have same number of valence electrons.
For example;
Let’s take the example of 1st group.
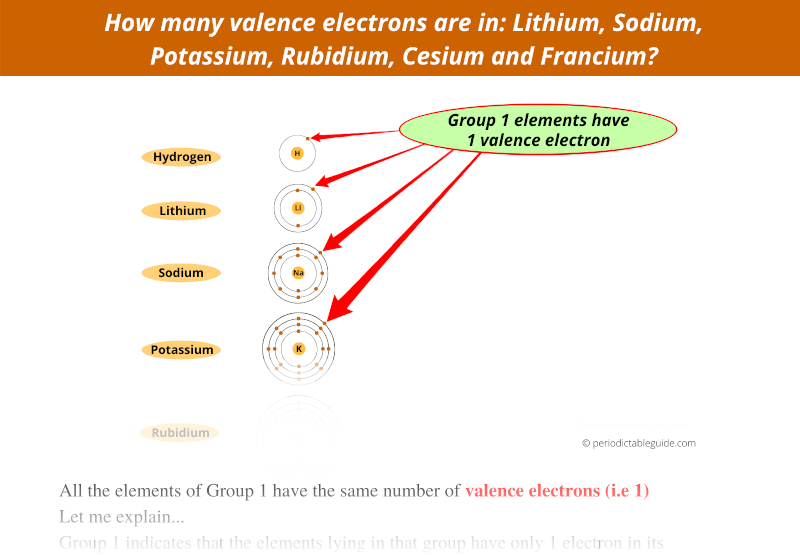
From the above image, you can see that all the group 1 elements have the same number of valence electrons (electrons in outermost orbit).
Similarly, elements lying in the other groups also show the same number of valence electrons.
Also visit: Basics of periodic table (Where you will come to know about the groups and periods, rows and columns as well as different types of elements present on the periodic table)
Quick look on Types of Elements on Periodic table
There are various types of elements on the Periodic table like;
- Metals
- Alkali metals
- Alkaline earth metals
- Transition metals
- Inner transition metals
- Nonmetals
- Metalloids
- Families on Periodic table
- Boron family
- Carbon family
- Nitrogen family
- Chalcogens
- Halogens
- Noble gases
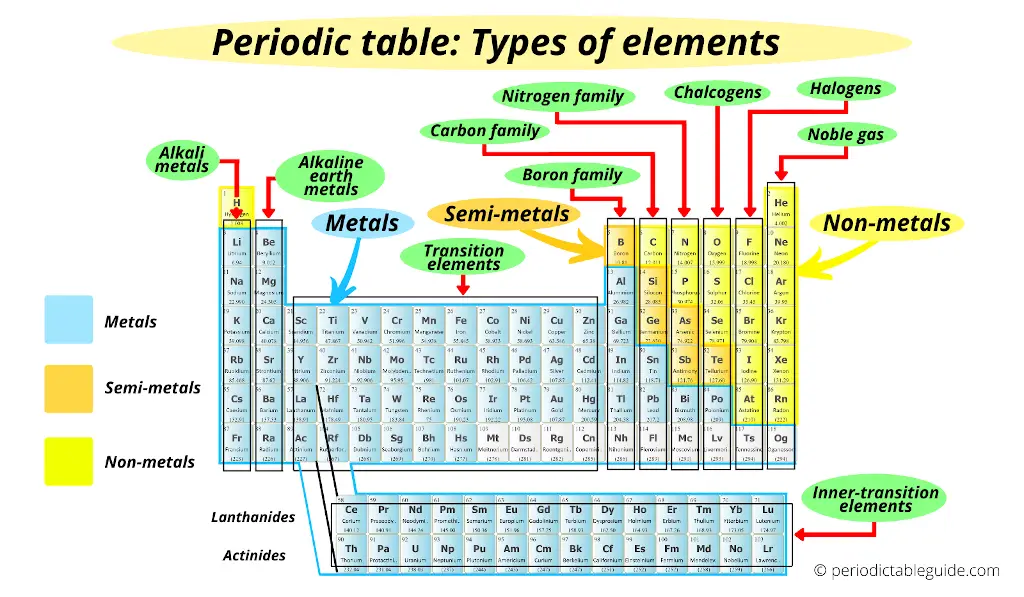
All these elements are shown in the above image.
But if you want to know more about the types of elements, you must visit this article “Types of elements on Periodic table” (where I have explained each of the types in very short and simple way)
Final words
There are total 118 elements on the Periodic table.
In order to classify and study each of them, it is necessary to arrange all the known elements according to their similarities.
In 1913, Henry Moseley arranged the known elements in the increasing order of their Atomic Number.
He arranged the elements in the grid of 18 columns and 7 rows.
And it was named as Modern Periodic table.
This Periodic table consists of vertical columns called groups and horizontal rows called periods.
There are various types of elements on the Periodic table like metals, nonmetals, metalloids, etc.
So this is how the Periodic table is arranged.
I hope now you have got a clear idea regarding the arrangement of elements in Periodic table.
If you have any doubts, feel free to ask me in the comments below.
Also let me know, has this article helped you or not?
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Suggested Important articles for you:
- Periodic table of elements (Detailed guide + HD image)
- Metals on the periodic table
- Nonmetals on the periodic table
- Metalloids on periodic table
- Halogens on periodic table
- Alkali metals on periodic table
- Alkaline earth metals on periodic table
- Transition metals on Periodic table
- Inner transition metals on periodic table
- All Periodic trends in periodic table
