
Have you ever wondered why are group 2 elements called alkaline earth metals?
You will get the exact answer here (with examples)
Here is your answer:
When alkaline earth metals (i.e Be, Mg, Ca, Sr, Ba and Ra) react with water, they form hydroxides which are alkaline in nature (or basis in nature).
Wait, I’ll give you an example to explain this.
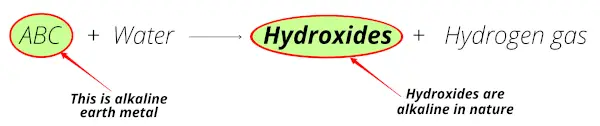
So when these alkaline earth metals react with water, they form hydroxides which are alkaline (or basic) in nature (means the solution has the pH > 7).
And also
All these metals are mostly obtained from the earth crust (in their oxides form) and these oxide minerals are stable to heat.
Hence,
Those metals which fulfills following two criteria:
- On reacting with water, they form hydroxides {Ba(OH)2 , Mg(OH)2 , Ca(OH)2 , etc} which are alkaline in nature and
- Their oxide minerals (BeO, beryl, MgO, magnesite, etc) are mostly found from the earth crust and are stable to heat.
are known as Alkaline Earth Metals.
Just see the bold word “alkaline” and “earth” in the above 2 points, you will easily get the exact idea why these metals are called alkaline earth metals.
Exception… Exception… Exception:
All the alkaline earth metals form an alkaline solution on reacting with water, but you know chemistry is all about exceptions. Hahaha…
Here also there is one exception.
Beryllium (Be) is the only alkaline earth metal which does not form an alkaline solution on reacting with water. The hydroxides of beryllium does not show alkaline behavior, but it shows amphoteric behavior. Hence, beryllium element of group 2 is not considered as alkaline earth metal.
Detailed explanation
If you haven’t understood the above concept of “why alkaline metals are called so?”, then read further.
I’ll show you some examples of chemical reaction.
It’s easy, don’t worry.
Keep reading…
I have mentioned that, when the alkaline earth metals react with water, they form hydroxides which are alkaline in nature.
Now let’s take an example to understand this;
Example 1
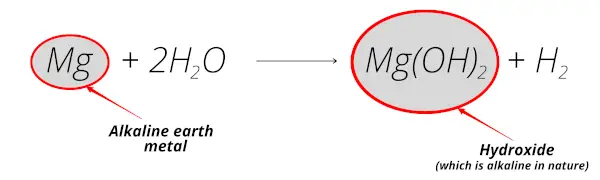
As shown in the above reaction, when magnesium reacts with water, it forms magnesium hydroxide which is alkaline in nature.
Plus this magnesium element is obtained from its mineral magnesite which is mostly found from the earth crust.

So magnesium is known as alkaline earth metal.
Example 2
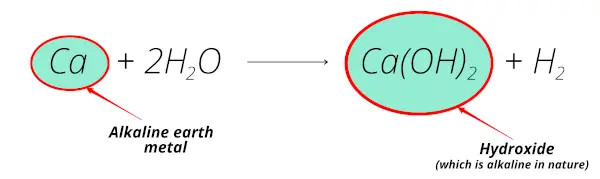
Now in this reaction also, you can see that when Calcium reacts with water, it forms calcium hydroxide which is alkaline in nature.
Also, the calcium element is obtained from the minerals like lime-stone, gypsum, calcite, etc which are mostly found from the earth crust.

Thus, calcium is called alkaline earth metal.
Example 3
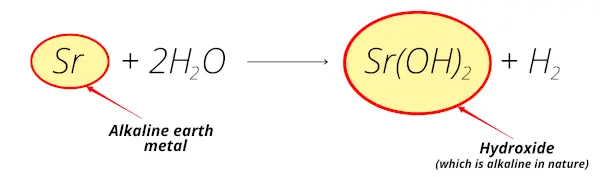
Another example,
Here you can see that Strontium reacts with water and forms strontium hydroxide which is also alkaline in nature.
And also the strontium element is extracted from the minerals like celestite and strontianite which are found from the earth crust.

So strontium is an alkaline earth metal.
Let me give you one more example. (Don’t worry, this is last)
Example 4
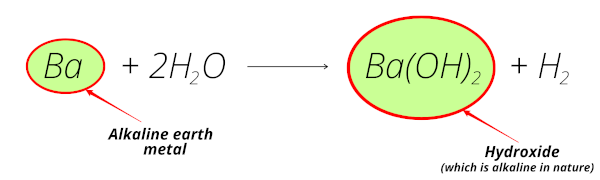
Here, you can see Barium reacts with water and forms barium hydroxide and this barium hydroxide is alkaline in nature.
Also, barium is obtained from the minerals like barytes, witherite, etc which are mostly found from the earth crust.

So barium is known as alkaline earth metal.
Conclusion
Hence from all the above chemical reactions, we have seen that all these metals form hydroxides which are alkaline (or basic) in nature, plus these metals are extracted from the minerals which are obtained from the earth crust.
So these group 2 elements (Be, Mg, Ca, Sr, Ba and Ra) are called alkaline earth metals.
I hope you have got the exact reason why alkaline earth metals are called so.
If you have any questions, feel free to comment down in the comment section.
Also let me know, has this article helped you or not?
Also visit: Why are group 1 elements called alkali metals?
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Suggested Important topics for you:
- Periodic table of elements (Detailed guide + HD image)
- Why are noble gases called so?
- Why transition metals are called so?
- What do periods on the periodic table represent?
- How are the elements arranged in the modern periodic table?
- Types of metals on Periodic table
- List of elements of Periodic table
- Metals on the periodic table
- Periodic trends in periodic table
References
Image credits:
Rob Lavinsky, iRocks.com – CC-BY-SA-3.0 / CC BY-SA
Manishwiki15 / CC BY-SA
James Petts from London, England / CC BY-SA
