
Do you know why are group 1 elements called alkali metals?
Here I will explain to you the exact reason why they are called so.
Here is the reason:
These are the alkali metals.
When these metals react with water, they form alkalis (i.e strong base).
Let me explain this to you with examples.
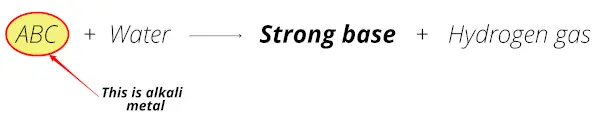
Here you can see that when these metals react with water, they form a strong base and releases the hydrogen gas.
In simple words, those metals which form a strong base (alkalis or alkaline solution) on reacting with water, are known as alkali metals.
Let’s understand this with a chemical reaction.
Example 1
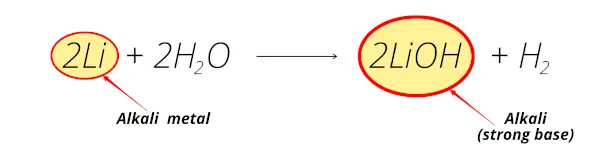
When lithium reacts with water, Lithium hydroxide (LiOH) is obtained which is alkaline in nature.
So for this reason, lithium is known as alkali metal.
Example 2
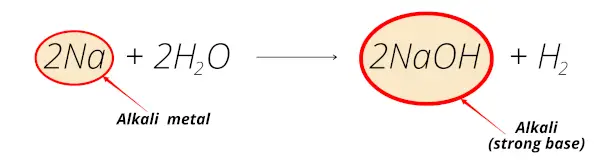
Now in this reaction, the sodium element reacts with water and forms sodium hydroxide (NaOH) which is alkaline solution.
Thus sodium is alkali metal.
Example 3
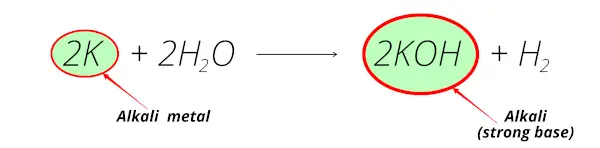
Here you can see that the potassium element reacts with water and forms potassium hydroxide (KOH) and this potassium hydroxide is an alkaline solution.
So potassium is an alkali metal.
Example 4
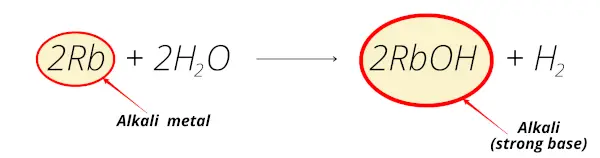
Similarly rubidium hydroxide (RbOH) is obtained when rubidium reacts with water.
And this rubidium hydroxide is alkaline in nature.
So rubidium is known as alkali metal.
Example 5
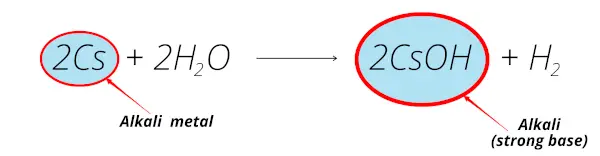
Let’s take the example of the cesium element.
When cesium reacts with water, it forms cesium hydroxide (CsOH) which is alkaline solution.
So because of this reason, cesium is also called alkali metal.
Now see, francium is a laboratory made element and it is highly reactive.
But the same reaction is predicted to occur with Francium (Fr). Thus francium is also alkali metal.
Conclusion
Hence, from the above reactions we have seen that all these metals form an alkaline solution on reacting with water.
So the group 1 elements are called alkali metals.
I hope you have understood the reason why alkali metals are called so.
If you have any questions, feel free to ask it in the comments below.
Also let me know, has this article helped you or not?
Also visit: Why are group 2 elements called alkaline earth metals?
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Suggested Important topics for you:
- Periodic table of elements (Detailed guide + HD image)
- Why are noble gases called so?
- Why transition metals are called so?
- What do periods on the periodic table represent?
- How are the elements arranged in the modern periodic table?
- What does group number and period number indicates about an element?
- Types of metals on Periodic table
- List of elements of Periodic table
- Metals on the periodic table
- Periodic trends in periodic table
