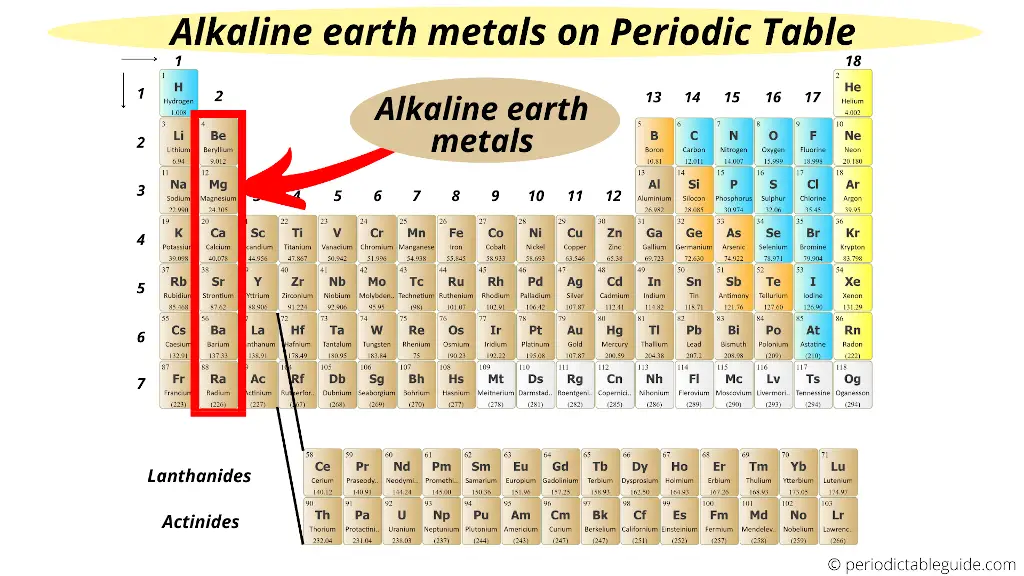
Alkaline earth metals are found in the 2nd group of the Periodic table.
This above image shows you where are alkaline earth metals found on the Periodic table.
Alkaline earth metals are located just next to the alkali metals.
Alkaline earth metals includes;
Beryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), Barium (Ba) and Radium (Ra).
But wait…
There are few more things you need to know about the alkaline earth metals, like;
- What exactly are alkaline earth metals?
- Alkaline earth metals list
- What do alkaline earth metals have in common?
- Alkaline earth metals facts
- How do alkaline earth metals occur in nature?
- Electronic configuration of alkaline earth metals
- Periodic trends of alkaline earth metals
So let’s finish this quickly.
What are Alkaline Earth Metals?

Have you ever wondered why alkaline earth metals are called so?
I’ll give you the exact answer for this.
Answer:
When these metals (Be, Mg, Ca, Sr, Ba and Ra) react with water, they form hydroxides which are alkaline in nature (or basis in nature).
Let me give you an example.
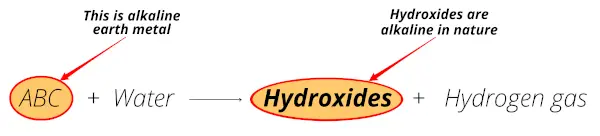
Above reaction shows that these metals form hydroxides which are alkaline (or basic having pH > 7) in nature.
Plus
These metals are mostly found from the earth crust (in their oxides form) and these oxide minerals are stable to heat.
Hence,
Those metals which fulfills following two criteria:
- On reacting with water, they form hydroxides {Ba(OH)2 , Mg(OH)2 , Ca(OH)2 , etc} which are alkaline in nature and
- Their oxide minerals (BeO, beryl, MgO, magnesite, etc) are mostly found from the earth crust and are stable to heat.
are known as Alkaline Earth Metals.
See the bold word “alkaline” and “earth” in the above 2 points, you will get the exact reason why they are called alkaline earth metals.
(Note: Beryllium (Be) is the only alkaline earth metal which does not form an alkaline solution on reacting with water. Hydroxides of beryllium does not show alkaline behavior, but it shows amphoteric behavior. Hence, beryllium element of group 2 is not considered as alkaline earth metal.)
Let me explain the above concept with an example.
I have mentioned that, when the alkaline earth metals react with water, they form hydroxides which are alkaline in nature. Lets take few examples.
Example 1
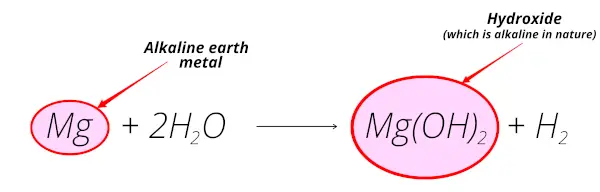
Magnesium reacts with water and forms magnesium hydroxide which is alkaline in nature.
Also, magnesium is obtained from the mineral magnesite which is mostly found from the earth crust.
So magnesium is alkaline earth metal.
Example 2
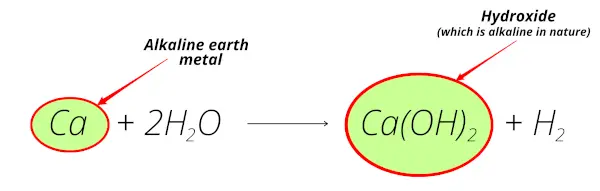
Calcium reacts with water and forms calcium hydroxide which is alkaline in nature.
Also, calcium is obtained from the minerals like lime-stone, gypsum, calcite, etc which are mostly found from the earth crust.
So calcium is alkaline earth metal.
Example 3
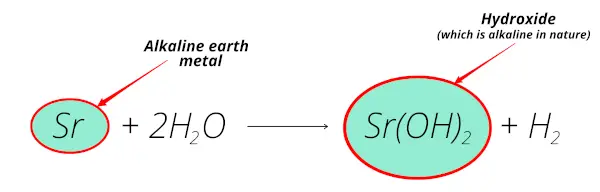
Strontium reacts with water and forms strontium hydroxide which is alkaline in nature.
Also, strontium is obtained from the minerals like celestite and strontianite which are found from the earth crust.
So strontium is alkaline earth metal.
Example 4
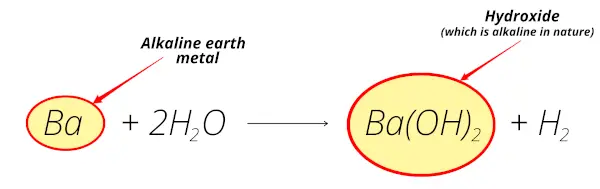
Barium reacts with water and forms barium hydroxide which is alkaline in nature.
Also, barium is obtained from the minerals like barytes, heavy spar, witherite, etc which are mostly found from the earth crust.
So barium is alkaline earth metal.
Hence from the above chemical reactions also, we have seen that all these metals form hydroxides which are alkaline (or basic) in nature, plus they are obtained from the minerals which are found from the earth crust.
So these elements (Mg, Ca, Sr, Ba and Ra) are called alkaline earth metals.
Alkaline earth metals list
List of alkaline earth metals with atomic number, symbol and name of element is mentioned in the table below.
| Atomic number | Symbol | Name of element |
| 4 | Be | Beryllium |
| 12 | Mg | Magnesium |
| 20 | Ca | Calcium |
| 38 | Sr | Strontium |
| 56 | Ba | Barium |
| 88 | Ra | Radium |
What do alkaline earth metals have in common?
Alkaline earth metals are similar in the following manner.
- All the alkaline earth metals have 2 valence electrons in their outermost orbit.
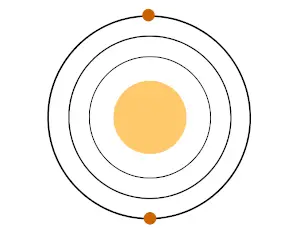
They lose these two electrons and form cation with +2 charge.
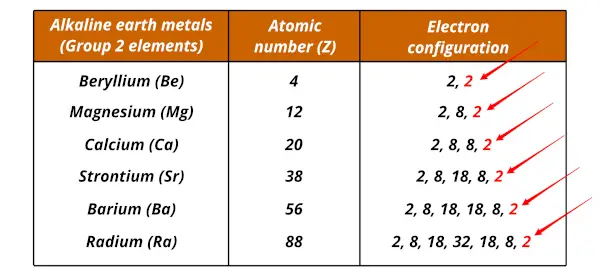
As you can see above that the alkaline earth metals have 2 electrons in the outermost shell.
They lose these 2 electrons during a chemical reaction which shows metallic character.
- All the alkaline earth metals are silvery-white, shiny and somewhat reactive.

Alkaline earth metals facts
Facts of alkaline earth metals are mentioned below.
- They are shiny.
- They react with Halogens and form hydrated halides.
- All the alkaline earth metals react with water (except beryllium).
- They show a coloured flame when they are heated strongly.
- Magnesium is also found in our food.
- They do not exist as a pure form in nature.
- Calcium is the element which is found in building materials like cement, concrete, marbles, etc.
- Strontium is known for its use in fireworks.
How do alkaline earth metals occur in nature?
Alkaline earth metals exist only in the compound form.
Reason?
They are the reactive metals and as they are reactive, they do not exist in pure form.
They also react with water and moisture of the air, if they are kept open.
As alkaline earth metals are reactive, they always occur in the compound form in nature.
For example;

Beryllium is found in its mineral beryl, chrysoberyl and phenacite.

Magnesium is found in its mineral magnesite.

Calcium is found in its mineral lime-stone, gypsum, calcite, etc..

Strontium is found from its mineral celestite and strontianite.

Barium is found from its mineral barytes, witherite, etc
Electronic configuration of alkaline earth metals
The electron configuration of alkaline earth metals is given in the table below.
| Element | Electronic configuration |
| Beryllium (Be) | [He] 2s2 |
| Magnesium (Mg) | [Ne] 3s2 |
| Calcium (Ca) | [Ar] 4s2 |
| Strontium (Sr) | [Kr] 5s2 |
| Barium (Ba) | [Xe] 6s2 |
| Radium (Ra) | [Rn] 7s2 |
Also visit:
1). Where are nonmetals on the periodic table?
2). Where are metalloids on the periodic table?
3). Periodic table labeled with metals, nonmetals and metalloids
Periodic trends of alkaline earth metals
Valency
The valency of the elements remains the same from top to bottom in a group.
Read more about: Valency trend in periodic table
Atomic size
The atomic size of the elements increases from top to bottom in a group.
Read more about: Atomic size trend in periodic table
Metallic character
Down the group, the atomic size increases. And as the atomic size increases, the electron will be lost easily. So the metallic character of alkaline earth metals increases from top to bottom in a group.
Read more about: Metallic character trend in periodic table
Electronegativity
As we move down in a group, Electronegativity of the alkaline earth metals decreases.
Read more about: Electronegativity trend in periodic table
Electron affinity
Electron affinity of the alkaline earth metals decreases down the group from top to bottom.
Read more about: Electron affinity trend in periodic table
Ionization energy
Ionization energy of the alkaline earth metals also decreases from top to bottom in a group.
Read more about: Ionization energy trend in periodic table
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Summary
In the very beginning of this article, I gave you a single image which clearly shows you where are alkaline earth metals found on the Periodic table.
Then we discussed about;
- Exact meaning of alkaline earth metals
- List of alkaline earth metals
- What do alkaline earth metals have in common?
- Alkaline earth metals facts
- How do alkaline earth metals occur in nature?
- Electronic configuration of alkaline earth metals and
- Periodic trends of alkaline earth metals
If you have read till the end, I am sure you have understood every single thing in detail.
If you have any questions. Leave a comment below in a comment section.
Also let me know whether this article helped you or not?
Suggested Important articles for you:
- Periodic table with everything (Important)
- Metals on the periodic table
- Nonmetals on periodic table
- Metalloids on periodic table
- Halogens on periodic table
- Alkali metals on periodic table
- Noble gases on periodic table
- Transition metals on Periodic table
- Inner transition metals on periodic table
- What do elements in the same group have in common?
References
Image credits:
Jurii, Hi-Res Images of Chemical Elements / CC BY
Rob Lavinsky, iRocks.com – CC-BY-SA-3.0 / CC BY-SA
Manishwiki15 / CC BY-SA
James Petts from London, England / CC BY-SA
