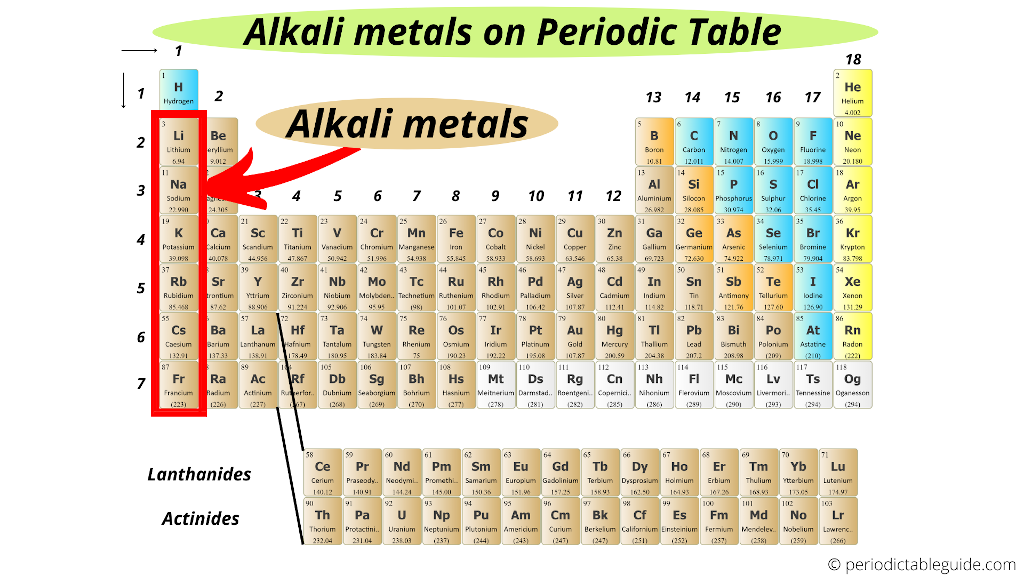
Alkali metals are located in group 1 on the left side of the Periodic table.
Alkali metals include: Lithium (Li), Sodium (Na), Potassium (K), Rubidium (Rb), Cesium (Cs) and Francium (Fr).
This above image clearly shows you where are Alkali metals located on the Periodic table.
But hold on…
There are lot more things you need to know about the Alkali metals like;
- What exactly are the alkali metals?
- Are alkali metals reactive?
- Why are alkali metals so reactive?
- Which alkali metal is most reactive?
- List of alkali metals
- Electronic configuration of alkali metals
- Periodic trends of alkali metals
If you want to skip to any of these topics, just click on the above links to navigate.
Let’s quickly finish this.
What are alkali metals on the periodic table?
Do you know why alkali metals are called alkali metals?
Here I will explain to you the exact reason behind this.
Reason:
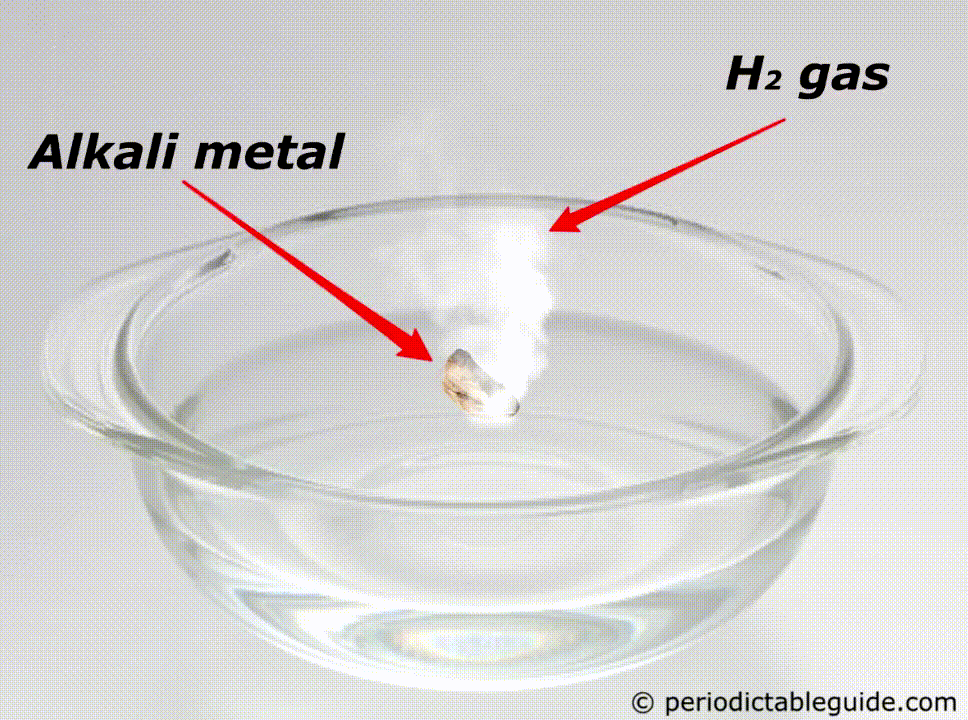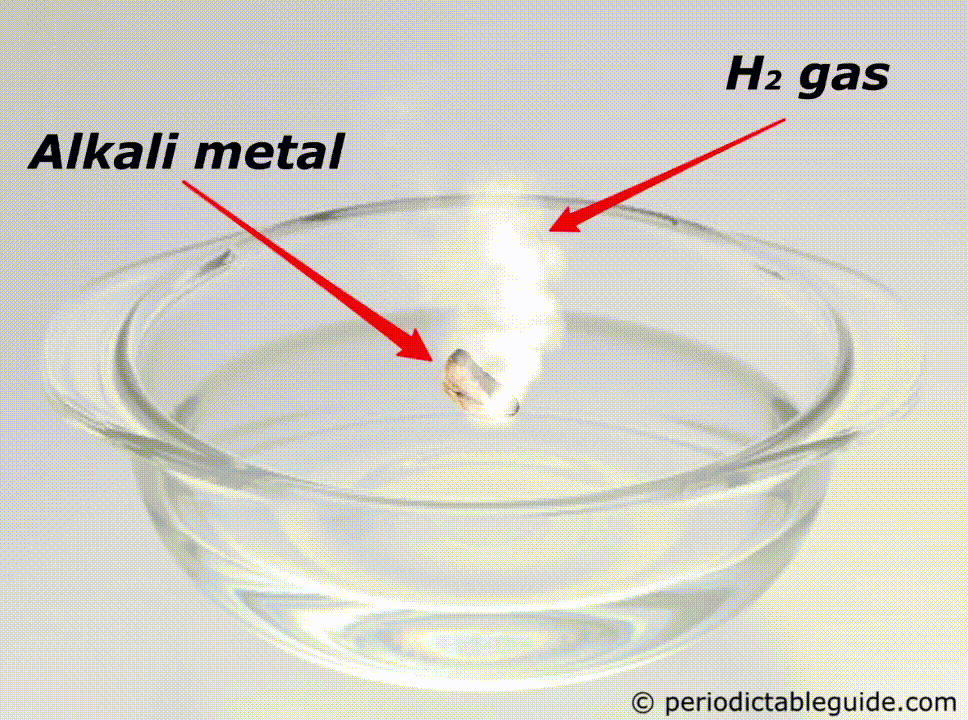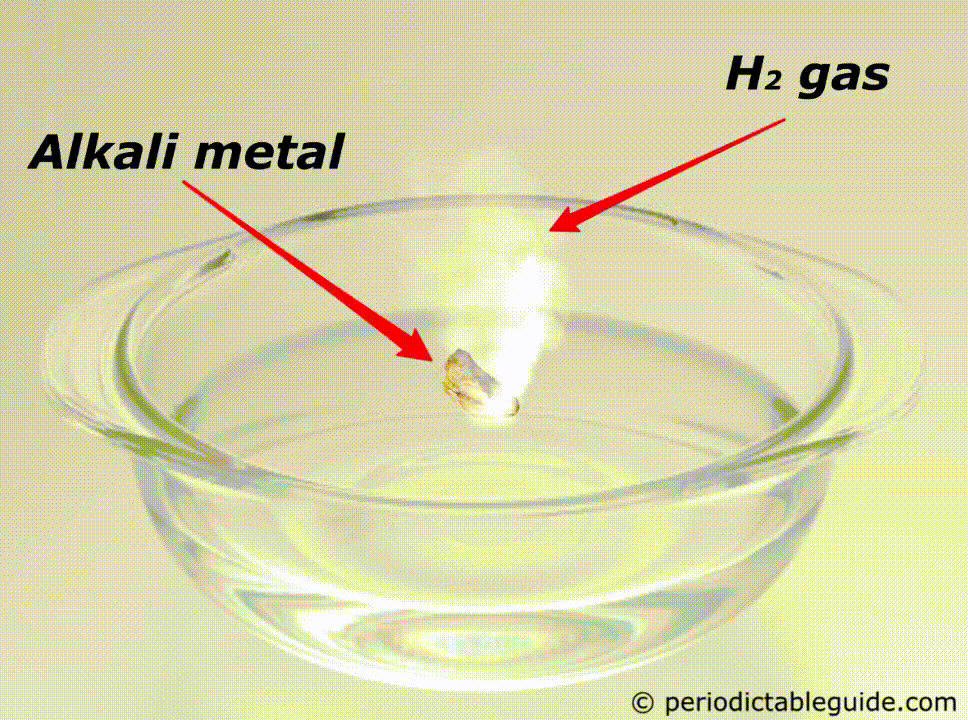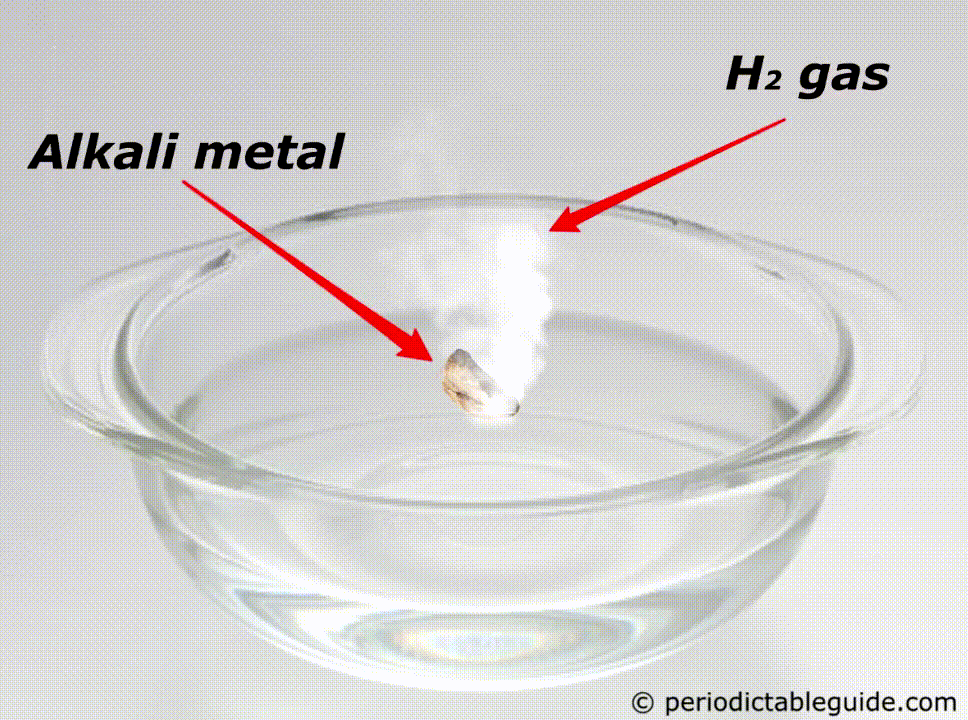
When these metals (Li, Na, K, Rb, Cs, Fr) react with water, they form alkalis (i.e strong base).
Don’t worry, I’ll explain to you with an example.
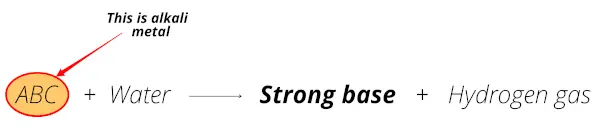
So those metals which form a strong base (alkalis or alkaline solution) on reacting with water, are known as alkali metals. Let me explain with examples.
Example 1
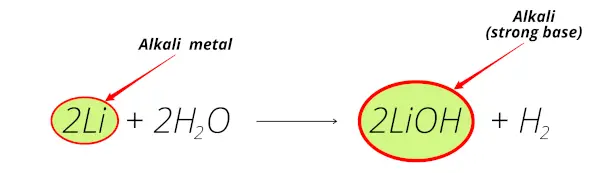
Lithium reacts with water and forms Lithium hydroxide (LiOH) which is alkaline solution.
Thus lithium is alkali metal.
Example 2
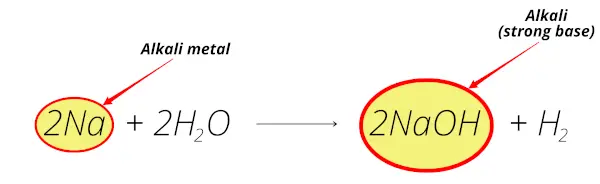
Sodium reacts with water and forms sodium hydroxide (NaOH) which is alkaline solution.
Thus sodium is alkali metal.
Example 3
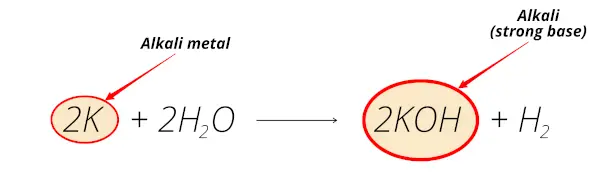
Potassium reacts with water and forms potassium hydroxide (KOH) which is alkaline solution.
Thus potassium is alkali metal.
Example 4
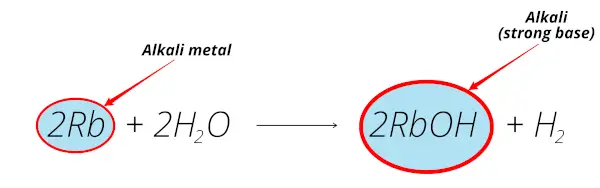
Rubidium reacts with water and forms rubidium hydroxide (RbOH) which is alkaline solution.
Thus rubidium is alkali metal.
Example 5
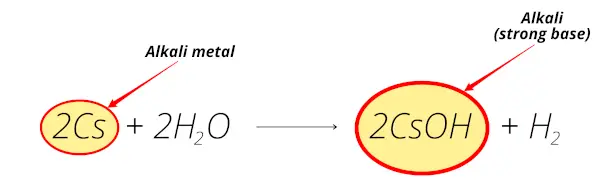
Cesium reacts with water and forms cesium hydroxide (CsOH) which is alkaline solution.
Thus cesium is alkali metal.
The same reaction is predicted to occur with Francium (Fr). Thus francium is also alkali metal.
Hence, from the above reactions we have seen that all these metals form an alkaline solution on reacting with water. So these metals are called alkali metals.
Are alkali metals reactive?

Yes, alkali metals are highly reactive.
When alkali metals are exposed to water, they instantly react with water to form alkalis (strong base) and thereby hydrogen gas is produced.
Facts about alkali metals
- Alkali metals are very reactive. So they are always found as a compound with other elements.
- Alkali metals are so reactive that they should not be kept in the open environment. They are highly reactive even if they come in contact with oxygen or moisture of the air.
- To avoid the contact with air or moisture, they are always kept in an airtight container under a mineral oil (or kerosene).
Why are alkali metals so reactive?
There are many reasons behind the reactivity of alkali metals. Let us discuss them one by one.
#1 Large atomic size
Larger atomic size of alkali metals indicates that there is a less attractive force between the nucleus and outermost electron.
Due to less attraction force, the alkali metals show a tendency to lose electrons easily. This makes them highly reactive.
Also read: Atomic size trend in periodic table.
#2 One valence electron
Alkali metals are present in the first group of Periodic table.
First group indicates that they have only one electron in their outermost orbit.
As only one electron is present in the outermost orbit, it is very easy to lose or donate this electron during a chemical reaction.
As it is easy to donate the electron, the chemical reaction is very easy and this makes alkali metals more reactive.
Also read: Valency trend in periodic table.
#3 Less ionization energy
Ionization energy is the minimum energy required to remove an electron from the gaseous atom or ion.
Now this ionization energy is very less for the elements located on the left side of the Periodic table. And as we move from left to right, the ionization energy increases.
Thus less ionization energy indicates that the atoms show a high tendency to lose electrons. So chemical reactions take place very fast and thus they are more reactive.
Also read: Ionization energy trend in periodic table.
#4 Lower boiling and melting point | Less density | Weak metallic bonds
Although alkali metals are solids, they have lower boiling point and melting point.
They have less intermolecular attractive force and have less density. Due to less intermolecular force and weak metallic bonds, they have more tendency to react with other elements. Hence they are more reactive.
Which alkali metal is most reactive?
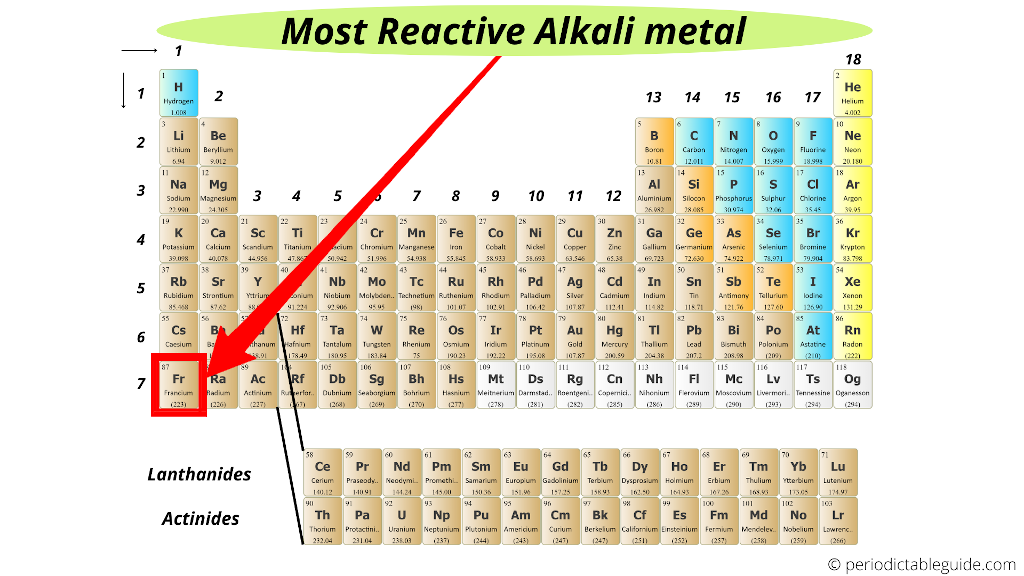
Francium (Fr) is the most reactive alkali metal on the Periodic table.
Here is an animation showing the reactivity of lithium, sodium and potassium with water.
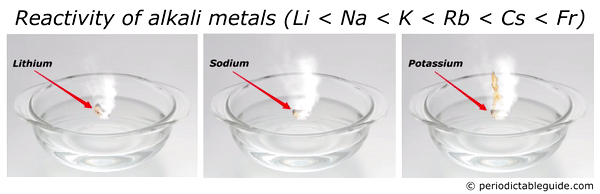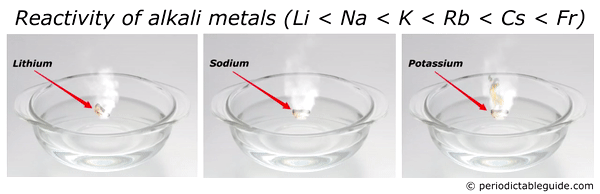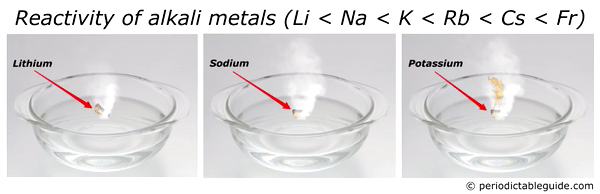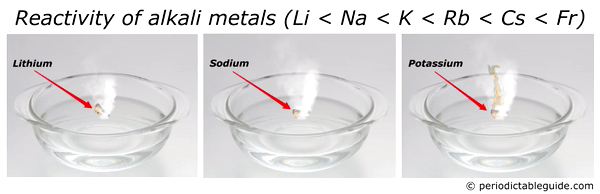
Reason?
Look, as we move down the group from top to bottom, the atomic size increases. Also alkali metals have only one electron in their outermost orbit.
Thus as the size of an atom increases, the loss of electron becomes very easy.
And hence the element with bigger atomic size (i.e Francium) is most reactive alkali metal.
(Note: Francium is a laboratory made element. It is available in a very less quantity. And hence for any practical purposes, cesium is considered as the most reactive metal in Periodic table. Cesium has high reactivity, but it is predicted that Francium would have even higher reactivity than that of cesium)
Also visit: Different types of metals on periodic table.
List of alkali metals
Here is a complete list of alkali metals present on the Periodic table with their atomic number, symbol and name of element.
| Atomic number | Symbol | Name of element |
| 3 | Li | Lithium |
| 11 | Na | Sodium |
| 19 | K | Potassium |
| 37 | Rb | Rubidium |
| 55 | Cs | Cesium |
| 87 | Fr | Francium |
Electronic configuration of alkali metals
Electronic configuration of alkali metals are shown below:
| Element | Electronic configuration |
| Lithium (Li) | [He] 2s1 |
| Sodium (Na) | [Ne] 3s1 |
| Potassium (K) | [Ar] 4s1 |
| Rubidium (Rb) | [Kr] 5s1 |
| Cesium (Cs) | [Xe] 6s1 |
| Francium (Fr) | [Rn] 7s1 |
Periodic trends of alkali metals
Valency
As we move down the group, the valence of the elements remains the same.
Read more about: Valency and its periodic trends
Atomic size
As we move down the group from top to bottom, the atomic size of the elements increases.
Read more about: Atomic size and its periodic trends
Metallic character
Down the group, the atomic size increases. And as the atomic size increases, the electron will be lost easily. So the metallic character of alkali metals increases down the group from top to bottom.
Read more about: Metallic character and its periodic trends
Electronegativity
Electronegativity of the alkali metals decreases from top to bottom in a group.
Read more about: Electronegativity and its periodic trends
Electron affinity
Electron affinity of the alkali metals decreases down the group from top to bottom.
Read more about: Electron affinity and its periodic trends
Ionization energy
Ionization energy of the alkali metals also decreases as we move down the group from top to bottom.
Read more about: Ionization energy and its periodic trends
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
Summary
In the beginning of this article, I showed you a single image which clearly shows you where are Alkali metals located on the Periodic table.
They are on the left side of the Periodic table (in group 1).
Then we discussed about;
- Meaning of alkali metals
- Reactivity of alkali metals
- Why are alkali metals so reactive?
- Most reactive alkali metal
- List of alkali metals
- Electronic configuration of alkali metals and
- Periodic trends of alkali metals
I hope all these topics about the alkali metals have helped you.
Let me know in the comments, whether this article has helped you or not?
Suggested Important articles for you:
- Periodic table (with everything you need to know) (Important)
- Metals on the periodic table
- Nonmetals on periodic table
- Metalloids on periodic table
- Halogens on periodic table
- Alkaline earth metals on periodic table
- Noble gases on periodic table
- Transition metals on Periodic table
- Inner transition metals on periodic table
- What do elements in the same group have in common?

