The groups in the Periodic table are also called Families because;
- The elements lying in the same group have same number of valence electrons, which results in their similar properties (like similar habits of all the family members.)
Explanation:
All the elements lying in the same groups have the same number of valence electrons.
Let me explain with an example.
Example 1:
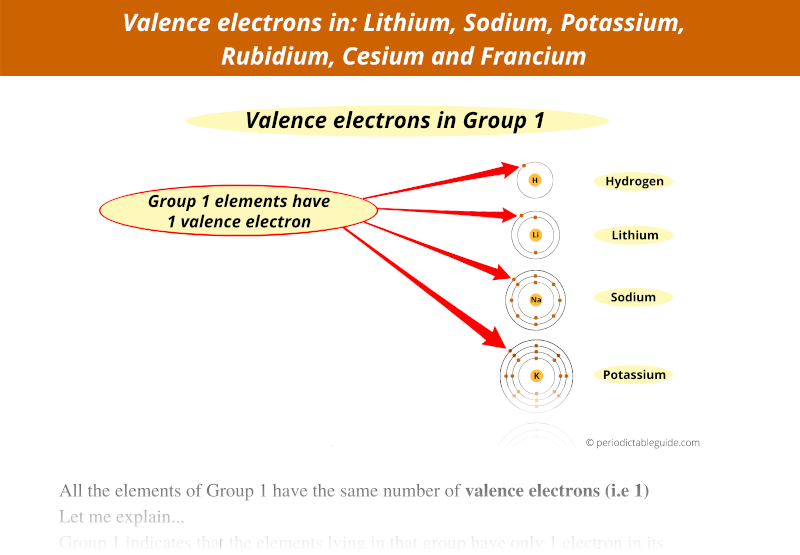
The elements of group 1 have 1 valence electron.
In other words, all the group 1 elements have 1 electron in their outermost orbit.
The important thing is that the chemical properties of any element depends on its valence electrons only.
Now, if the elements have same number of valence electrons, then their chemical properties will be similar.
And if the elements have different number of valence electrons, then they will have different chemical properties.
Let us take the example of group 1 elements only.
As I mentioned earlier, all the group 1 elements have 1 valence electron in their outermost orbit.
Because of this, they have a high tendency to lose the electron. (They loses one electron and becomes stable.)
These elements of group 1 are highly reactive and they always exist in the compound form with other elements.
They react with water and forms Alkaline solution.
Hence they are also called alkali metals.
Read more about: Alkali metals and how they form Alkaline solution?
Thus because of the similar outer electrons of group 1 elements, they have similar chemical properties.
And as they have similar properties, they behave like the members of the same family.
Example 2:
Let’s take the example of last group, (i.e group 18).
All the group 18 elements have 8 valence electrons. (Note: Helium has 2 valence electrons.)
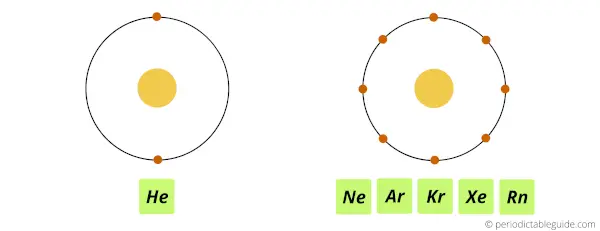
Because of this, they are chemically inert. In other words, all the group 18 elements do not easily react with any other elements. Hence they are chemically inert.
Thus, because of the same number of electrons present in the outermost orbit, elements of the same group show a similar behavior or similar chemical properties.
Read more about: What are noble gases and why are they called so?
Hence the groups in the Periodic table are also called Families.
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
