The reason why there are 7 periods in the Periodic table is mentioned below:
- Periods indicates the number of orbits. And out of the 118 known elements, no element is having more than 7 orbits.
- Element/s with atomic number greater than 118 is/are not discovered yet, thus there are only 7 periods in Periodic table.
(Note: Right now, there are 7 periods in the Periodic table. In future, there may be more than 7 periods in the Periodic table after the discovery of new elements.)
Let me explain all these things to you in a simple way.
You know that the period number indicates the number of orbits in an element.
For example, Lithium and helium are in period 1. So they have only 1 orbit (or shell).
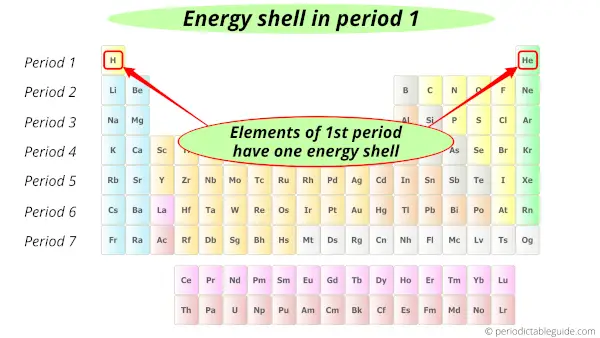
Similarly,
Period 2 elements have 2 orbits.
Period 3 elements have 3 orbits.
.
.
.
Period 7 elements have 7 orbits.
All the known elements are adjusted in these 7 periods.
There is no element present till now, whose electrons enter in the 8th orbit.
Hence out of all the natural and synthetic elements (laboratory made elements), there is no such element which shows the electrons in 8th orbit.
In other words, elements with atomic number higher than 118 does not exist right now. So there are only 7 periods till today.
If new elements with atomic number higher than 118 will be discovered in future, then it will be placed in the 8th period of the Periodic table.
In 1969, Glenn T Seaborg had suggested the Periodic table with 8 rows having a new g-block, which may include many more elements (even more than the elements in 7th period, because of new g-orbitals in the atoms.)
But this is just a hypothetical approach. The fact is that there is no element discovered yet whose atomic number is higher than 118.
So just chill for now (2022) !!!
I hope you have clearly understood the reason why there are only 7 periods on the Periodic table.
Let me know in the comments, whether this has helped you or not?
Explore our New Interactive Periodic Table (with Rotating Bohr Models and More)

Details about this Periodic table:
- Access detailed info on all elements: atomic mass, electron configurations, charges, and more.
- View rotating Bohr models for all 118 elements.
- Get a free HD image of the Periodic Table.
Note: For future use, bookmark this Periodic table or visit “PeriodicTableGuide.com”
